Table 2. List of species of insect pests that are susceptible to major entomopathogenic nematodes
Species of insect pests
|
Entomopathogenic nematode species
|
Publications
(See below)
|
| Apopka weevil, Citrus root weevil or Sugarcane borer Diaprepes abbreviatus |
Heterorhabditis georgiana, H. indica, H. zealandica, Steinernema carpocapsae, S. diaprepesi, S. riobrave |
1-13 |
| Armyworms, Helicoverpa (Heliothis) armigera, Spodoptera exigua, S. frugiperda |
H. amazonensis, H. indica S. arenarium, S. carpocapsae, S. glaseri |
14-18 |
| Billbugs, Sphenophorus purvulus, S. levis |
H. bacteriophora, S. brazilense, S. carpocapsae |
19-20 |
| Black vine weevil, Otiorhynchus salcatus |
H. bacteriophora, H. downesi, H. megidi.S. carpocapsae, S. feltiae, S. glaseri, S. kraussei |
21-26 |
| Bluegrass weevil, Listronotus maculicollis |
H. bacteriophora, S. carpocapsae |
27-29 |
| Carpenter worms, Cossus cossus |
S. weiseri |
30 |
| Carrot weevil, Listronotus oregonensis |
H. bacteriophora, H. megidi, S. feltiae, S. carpocapsae, S. riobrave, feltiae |
31-32 |
| Cat fleas, Ctenocephalides felis |
S. carpocapsae |
33-34 |
| Chestnut weevil, Curculio elephas |
H. bacteriophora, S. carpocapsae, S. feltiae, S. siamkayai, S. weiseri |
35-37 |
| Chinch bugs, Blissus sp. |
Unknown species |
38 |
| Citrus root weevil, Pachnaeus litus |
S. carpocapsae |
39-41 |
| Clover root weevil, Sitona hispidulus |
H. bacteriophora |
42-43 |
| Codling moth, Cydia pomonella |
H. bacteriophora, H. zealandica, S. carpocapsae, S. feltiae, S. kraussei |
44-56 |
| Crane flies, Tipula paludosa |
H. marelatus, H. megidis, S. carpocapsae, S. feltiae |
57-58 |
| Cucurbit beetle, Diabrotica speciosa |
H. amazonensis, S. glaseri |
59 |
| Cutworms, Agrotis ipsilon, A. segetum |
H. bacteriophora, H. georgiana, H. indica H. Mexicana, S. carpocapsae, S. feltiae, S. riobrave |
60-65 |
| Diamondback moth, Plutella xylostella |
Heterorhabditis sp., Rhabditis blumi, S. carpocapsae |
66-70 |
| Egyptian cotton leaf worm, Spodoptera littoralis |
H. bacteriophora, S. glaseri, S. feltiae, S. carpocapsae, S. kraussei, S. riobrave |
71-73 |
| Fall webworms, Hyphantria cunea |
H. bacteriophora, S. feltiae |
74 |
| Filbertworm, Cydia latiferreana |
S. carpocapsae, S. kraussei |
75-76 |
| Flea beetles, Phyllotreta striolata, P. cruciferae |
H. bacteriophora, H. indica, H. megidi, S. carpocapsae, S. feltiae, S. pakistanense |
77-79 |
| Fungus gnats, Bradysis spp. |
H. bacteriophora, H. indica, H. zealandica, S. anomali, S. carpocapsae, S. feltiae, S. riobrave |
80-84 |
| House flies, Musca domestica |
H. bacteriophora, H. megidi, S. carpocapsae, S. feltiae, S. scapterisci |
85-89 |
| Japanese beetle, Popillia japonica, P. unipuncta |
H. bacteriophora, H. indica, H. marelata, H. megidis, H. zealandica, S. anomaly, S. carpocapsae, S. feltiae, S. glaseri, S. kushidai, S. minuta, S. scapterisci, S. scarabae, S. riobrave |
90-99 |
| Leaf minors, Liriomyza bryoniae, L. trifolii, L. huidobrensis |
S. carpocapsae, S. feltiae |
100-107 |
| Leopard moth, Zeuzera pyrina |
H. bacteriophora, H. heliothidis, S. carpocapsae |
108 |
| Mediterranean fruit fly, Ceratitis capitata |
H. bacteriophora, H. zealandica, S. carpocapsae, S. feltiae, S. khoisanae, S. siamkayai, S. weiseri |
109-116 |
| Mole crickets, Scapteriscus vicinus |
S. carpocapsae, S. riobravis, S. scapterisci |
117-131 |
| Navel orangeworm, Amyelois transitella |
S. carpocapsae |
132 |
| Peach borer, Synanthedon exitiosa |
H. bacteriophora, S. carpocapsae, S. riobrave |
133-134 |
| Pecan weevil, Curculio caryae, C. hicoriae |
H. bacteriophora, H. indica, H. megidis, H. Mexicana, S. carpocapsae, S. riobrave |
135-143 |
| Pine weevil, Hylobius abietis |
H. downesi, H. megidis, S. carpocapsae, S. feltiae |
144-148 |
| Plum weevil, Conotrachelus nenuphar |
H. bacteriophora, S. carpocapsae, S. feltiae, S. riobrave |
149-154 |
| Shore flies, Scatella stagnalis, S. tenuicosta |
H. bacteriophora, H. megidis, S. anomaly, S. arenarium, S. carpocapsae, S. feltiae |
155-158 |
| Sod webworm, Herpetogramma phaeopteralis |
S. carpocapsae, S. feltiae |
159 |
| Spruce webworm, Cephalcia abietis |
S. feltiae |
160 |
| Stable fly, Stomoxys calcitrans |
H. heliothidis, S. glaseri |
161 |
| Stored grain pests: Indian meal moth (Plodia interpunctella), Mediterranean flour moth (Ephestia kuehniella), Sawtoothed grain beetle (Oryzaephilus surinamensis), Mealworm (Tenebrio molitor), Red flour beetle (Tribolium castaneum), Warehouse beetle (Trogoderma variabile) |
H. bacteriophora, H. megidis, S. carpocapsae, S. feltiae |
162-168 |
| Strawberry root borer, Nemocestes incomptus |
S. carpocapsae |
169 |
| Strawberry root weevil, Otiorhynchus ovatus, O. dubius strom, Ptiorhynchus ovatus |
H. bacteriophora, H. marelatus, S. carpocapsae |
170-173 |
| Strawberry crown moth, Synanthedon bibionipennis |
H. bacteriophora, S. carpocapsae |
174 |
| Tick, Rhipicephalus (Boophilus) microplus |
H. amazonensis, S. carpocapsae, S. glaseri |
175-179 |
| Western flower thrips, Frankliniella occidentalis, Thrips palmi |
H. bacteriophora, H. indica, S. arenarium, S. bicornutum, S. carpocapsae, S. feltiae, Thripinema nicklewoodi |
180-186 |
| Western corn rootworm, Diabrotica virgifera virgifera |
H. bacteriophora, S. carpocapsae |
187-189 |
| White flies, Bemisia tabaci, Trialeurodes vaporariorum |
H. bacteriophora, H. megidis, S. feltiae |
190-194 |
| White grub (Summer Chafer), Amphimallon solstitiale |
H. bacteriophora |
195 |
| White grub (Oriental beetle), Anomala orientalis, Exomala orientalis, Blitopertha orientalis |
H. bacteriophora, H. megidis, H. zealandica, S. carpocapsae, S. glaseri, S. longicaudum, S. scarabae |
196-216 |
| White grub, Costelytra zealandica |
H. bacteriophora, S. glaseri |
217 |
| White grub (June Bettle), Cotinus nitida |
H. bacteriophora, S. carpocapsae, S. feltiae, S. glaseri, S. scarabae |
218-220 |
| White grub, Cyclocephala borealis, C. hirta, C. lurida, C. pasadenae |
H. bacteriophora, H. indica, H. marelata, H. megidis, H. zealandica, S. carpocapsae, S. feltiae, S. glaseri, S. kushidai, S. riobrave, S. scarabae |
221-227 |
| White grub, Hoplia philanthus |
H. bacteriophora, H. indica, H. megidis, S. arenarium, S. carpocapsae, S. feltiae, S. glaseri, S. scarabaei |
228-232 |
| White grub, Melolontha melolontha |
H. bacteriophora, H. marelata, H. megidis, S. arenaria, S. feltiae, S. glaseri, S. riobrave |
233-235 |
| White grub, Ataenius spretulus |
H. bacteriophora, S. glaseri, S. scarabae |
236-237 |
| White grub (Asiatic garden beetle), Maladera castanea |
H. bacteriophora, S. glaseri, S. scarabae |
238-242 |
| White grubs, Phyllophaga anxia, P. bicolor, P. congrua, P. crinita, P. georgiana, P. hirticula, P. menetriesi |
H. bacteriophora, H. heliothidis, H. zealandica, S. carpocapsae, S. feltiae, S. glaseri, S. riobrave, S. scarabae |
243-250 |
| White grub, Rhizotrogus majalis |
H. bacteriophora, H. megidis, H. zealandica, S. carpocapsae, S. feltiae, S. glaseri, S. scarabae |
251-255 |
| Fuller rose beetle, Asynonychus godmani |
S. carpocapsae |
256 |
| Chive gnat, Bradysia odoriphaga |
H. bacteriophora, H. indica, H. megidis, S. ceratophorum, S. feltiae, S. hebeiense, S. litorale |
257-258 |
Publications:
Apopka weevil, Diaprepes abbreviatus
1.
Ali, J.G., Alborn, H.T. and Stelinski, L.L. 2010. Subterranean herbivore-induced volatiles released by citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes. Journal of Chemical Ecology. 36: 361-368.
2. Bullock, R.C., Pelosi, R.R. and Killer, E.E. 1999. Management of citrus root weevils (Coleoptera: Curculionidae) on Florida citrus with soil-applied entomopathogenic nematodes (Nematoda: Rhabditida). Florida Entomologist. 82: 1-7.
3. Duncan, L. W., Stuart, R. J., El-Borai, F. E., Campos-Herrera, R., Pathak, E., Giurcanu, M. and Graham, J. H. 2013. Modifying orchard planting sites conserves entomopathogenic nematodes, reduces weevil herbivory and increases citrus tree growth, survival and fruit yield. Biological Control 64: 26-36.
4. Duncan, L.W and McCoy, C.W. 1996. Vertical distribution in soil, persistence, and efficacy against citrus root weevil (Coleoptera: Curculionidae) of two species of entomogenous nematodes (Rhabditida: Steinernematidae; Heterorhabditidae). Environmental Entomology. 25: 174-178.
5. Duncan, L.W. McCoy, C.W. and Terranova, A.C. 1996. Estimating sample size and persistence of entomogenous nematodes in sandy soils and their efficacy against the larvae of Diaprepes abbreviatus in Florida. Journal of Nematology. 28: 56-67.
6. El-Borai, F.E., Stuart, R.J., Campos-Herrera, R., Pathak, E. and Duncan, L.W. 2012. Entomopathogenic nematodes, root weevil larvae, and dynamic interactions among soil texture, plant growth, herbivory, and predation. Journal of Invertebrate Pathology 109: 134-142.
7. Kaspi, R., Ross, A., Hodson, A.K., Stevens, G.N., Kaya, H.K. and Lewis, E.E. 2010. Foraging efficacy of the entomopathogenic nematode Steinernema riobrave in different soil types from California citrus groves. Applied Soil Ecology 45: 243-253.
8. Schroeder, W.J. 1992. Entomopathogenic nematodes for control of root weevils of citrus. Florida Entomologist 75: 563-567.
9. Shapiro, D.I. and McCoy, C.W. 2000. Susceptibility of Diaprepes abbreviatus (Coleoptera: Curculionidae) larvae to different rates of entomopathogenic nematodes in the greenhouse. Florida Entomologist. 83: 1-9.
10. Shapiro, D.I. and McCoy, C.W. 2000. Effects of culture method and formulation on the virulence of Steinernema riobrave (Rhabditida: Steinernematidae) to Diaprepes abbreviatus (Coleoptera: Curculionidae). Journal of Nematology 32: 281-288.
11. Shapiro, D.I., Cate, J. R., Pena, J., Hunsberger, A. and McCoy, C.W. 1999. Effects of temperature and host age on suppression of Diaprepes abbreviatus (Coleoptera: Curculionidae) by entomopathogenic nematodes. Journal of Economic Entomology. 92: 1086-1092.
12. Shapiro-Ilan, D.I., Mbata, G.N., Nguyen, K.B., Peat, S.M., Blackburn, D. and Adams, B.J. 2009. Characterization of biocontrol traits in the entomopathogenic nematode Heterorhabditis georgiana (Kesha strain), and phylogenetic analysis of the nematode's symbiotic bacteria. Biological Control 51: 377-387.
13. Shapiro-Ilan, D.I., Morales-Ramos, J.A., Rojas, M.G. and Tedders, W.L. 2010. Effects of a novel entomopathogenic nematode-infected host formulation on cadaver integrity, nematode yield, and suppression of Diaprepes abbreviatus and Aethina tumida. Journal of Invertebrate Pathology. 103: 103-108.
Armyworms, Heliothis armiger, Spodoptera exigua, S. frugiperda
14. Andalo, V., Santos, V., Moreira, G.F., Moreira, C., Freire, M. and Moino, A. 2012. Movement of Heterorhabditis amazonensis and Steinernema arenarium in search of corn fall armyworm larvae in artificial conditions. Scientia Agricola 69: 226-230.
15. Ansari, M.A., Waeyenberge, L. and Moens, M. 2007. Natural occurrence of Steinernema carpocapsae, Weiser, 1955 (Rhabditida: Steinernematidae) in Belgian turf and its virulence to Spodoptera exigua (Lepidoptera: Noctuidae). Russian Journal of Nematology 15: 21-24.
16. Kim, J. and Kim, Y. 2011. Three metabolites from an entomopathogenic bacterium, Xenorhabdus nematophila, inhibit larval development of Spodoptera exigua (Lepidoptera: Noctuidae) by inhibiting a digestive enzyme, phospholipase A (2). Insect Science 18: 282-288.
17. Negrisoli, A.S., Garcia, M.S., Negrisoli, C.R.C.B., Bernardi, D. and da Silva, A. 2010. Efficacy of entomopathogenic nematodes (Nematoda: Rhabditida) and insecticide mixtures to control Spodoptera frugiperda (Smith, 1797) (Lepidoptera: Noctuidae) in corn crops. Crop Protection. 29: 677-683.
18. Salvadori, J.D., Defferrari, M.S., Ligabue-Braun, R., Lau, E.Y., Salvadori, J.R. and Carlini, C.R. 2012. Characterization of entomopathogenic nematodes and symbiotic bacteria active against Spodoptera frugiperda (Lepidoptera: Noctuidae) and contribution of bacterial urease to the insecticidal effect. Biological Control 63: 253-263.
Billbugs, Sphenophorus spp.
19. Georgis, R., Koppenhofer, A.M., Lacey, L.A., Belair, G., Duncan, L.W., Grewal, P.S., Samish, M., Tan, L., Torr, P. and van Tol, R.W.H.M. 2006. Successes and failures in the use of parasitic nematodes for pest control. Biological Control 38: 103-123.
20. Giometti, F.H.C., Leite, L.G., Tavares, F.M., Schmit, F.S., Batista, A. and Dell'Acqua, R. 2011. Virulence of entomopathogenic nematodes (Nematoda: Rhabditida) against Sphenophorus levis (Coleoptera: Curculionidae). Bragantia 70: 81-86.
Black vine weevil, Otiorhynchus sulcatus
21. Ansari, M. A. and Butt, T. M. 2011. Effect of potting media on the efficacy and dispersal of entomopathogenic nematodes for the control of black vine weevil, Otiorhynchus sulcatus (Coleoptera: Curculionidae). Biological Control 58: 310-318.
22. Ansari, M.A., Shah, F.A. and Butt, T.M. 2008. Combined use of entomopathogenic nematodes and Metarhizium anisopliae as a new approach for black vine weevil, Otiorhynchus sulcatus control. Entomologia Experimentalis Et Applicata 129: 340-347.
23. Ansari, M.A., Shah, F.A. and Butt, T.M. 2010. The entomopathogenic nematode Steinernema kraussei and Metarhizium anisopliae work synergistically in controlling overwintering larvae of the black vine weevil, Otiorhynchus sulcatus, in strawberry growbags. Biocontrol Science and Technology. 20: 99-105.
24. Haukeland, S. and Lola-Luz, T. 2010. Efficacy of the entomopathogenic nematodes, Steinernema kraussei and Heterorhabditis megidis against the black vine weevil Otiorhynchus sulcatus in open field-grown strawberry plants. Agricultural and Forest Entomology.12363-369.
25. Lola-Luz, T. and Downes, M. 2007. Biological control of black vine weevil Otiorhynchus sulcatus in Ireland using Heterorhabditis megidis. Biological Control 40: 314-319.
26. Susurluk, A. and Ehlers, R.U. 2008. Sustainable control of black vine weevil larvae, Otiorhynchus sulcatus (Coleoptera: Curculionidae) with Heterorhabditis bacteriophora in strawberry. Biocontrol Science and Technology 18: 635-640.
Bluegrass weevil, Listronotus maculicollis
27. McGraw, B.A. and Koppenhofer, A.M.2008. Evaluation of two endemic and five commercial entomopathogenic nematode species (Rhabditida: Heterorhabditidae and Steinernematidae) against annual bluegrass weevil (Coleoptera: Curculionidae) larvae and adults. Biological Control 46: 467-475.
28. McGraw, B.A. and Koppenhofer, A.M.2009. Population dynamics and interactions between endemic entomopathogenic nematodes and annual bluegrass weevil populations in golf course turfgrass. Applied Soil Ecology 41: 77-89.
29. McGraw, B.A., Vittumb, P.J. Cowlesc, R.S.and Koppenhoumlfera, A.M. 2010. Field evaluation of entomopathogenic nematodes for the biological control of the annual bluegrass weevil, Listronotus maculicollis (Coleoptera: Curculionidae), in golf course turfgrass. Journal Biocontrol Science and Technology. 20: 149 – 163.
Carpenter worms, Cossus cossus
30. Bazman, I., Ozer, N., and Hazir, S. 2008. Bionomics of the entomopathogenic nematode, Steinernema weiseri (Rhabditida: Steinernematidae). Nematology 10: 735-742.
Carrot weevil, Listronotus oregonensis
31. Belair, G. and Boivin, G. 1995. Evaluation of Steinernema-carpocapsae weiser for control of carrot weevil adults, Listronotus-oregonensis (leconte) (coleopteran: curculionidae), in organically grown carrots. Biocontrol Science and Technology 5: 225-231.
32. Miklasiewicz, T.J., Grewal, P.S., Hoy, C.W. and Malik, V.S. 2002. Evaluation of entomopathogenic nematodes for suppression of carrot weevil. Biocontrol 47: 545-561.
Cat fleas, Ctenocephalides felis
33. Henderson, G., Manweiler, S.A., Lawrence, W.J., Tempelman, R.J.and Foil, L.D. 1995. The effects of Steinernema-carpocapsae (weiser) application to different life stages on adult emergence of the cat flea Ctenocephalides-felis (bouche). Veterinary Dermatology 6: 159-163.
34. Silverman J.S., Platzer, E.G. and M.K. Rust, M.K. 1982. Infection of the cat flea, Ctenocephalides felis (Bouche) by Neoaplectana carpocapsae Weiser. Journal of Nematology 14: 394-397.
Chestnut weevil, Curculio elephas
35. Karagoz, M., Gulcu, B., Hazir, S. and Kaya, H.K. 2009. Laboratory evaluation of Turkish entomopathogenic nematodes for suppression of the chestnut pests, Curculio elephas (Coleoptera: Curculionidae) and Cydia splendana (Lepidoptera: Tortricidae). Biocontrol Science and Technology. 19: 755-768.
36. Kepenekci, I., Gokce, A. and Gaugler, R. 2004. Virulence of three species of entomopathogenic nematodes to the chestnut weevil, Curculio elephas (Coleoptera: Curculionidae). Nematropica 34: 199-204.
37. Raja, R.K., Sivaramakrishnan, S. and Hazir, S. 2011. Ecological characterisation of Steinernema siamkayai (Rhabditida: Steinernematidae), a warm-adapted entomopathogenic nematode isolate from India. Biocontrol 56: 789-798.
Chinch bugs Bilssus spp.
38. Baxendale, F.P., A.P. Weinhold, and T.P. Riordan. 1994. Control of buffalograss chinch bugs with Beauvaria bassiana and entomopathogenic nematodes, 1993. Nebraska Insect Management and Insecticide Efficacy Reports, Dept. of Entomology Report No. 18, Univ. of Nebr., p. 43.
Citrus root weevil, Pachnaeus litus
39. Bullock, R.C., Pelosi, R.R. and Killer, E.E. 1999. Management of citrus root weevils (Coleoptera: Curculionidae) on Florida citrus with soil-applied entomopathogenic nematodes (Nematoda: Rhabditida). Florida Entomologist 82: 1-7.
40. Duncan, L.W., Graham, J.H., Dunn, D.C., Zellers, J., McCoy, C.W. and Nguyen, K. 2003. Incidence of endemic entomopathogenic nematodes following application of Steinerema riobrave for control of Diaprepes abbreviates. Journal of Nematology 35: 178-186.
41. Schroeder, W.J. 1992. Entomopathogenic nematodes for control of root weevils of citrus. Florida Entomologist 75: 563-567.
Clover root weevil, Sitona hispidulus
42. Loya, L.J. and Hower, A.A. 2002. Population dynamics, persistence, and efficacy of the entomopathogenic nematode Heterorhabditis bacteriophora (Oswego strain) in association with the clover root curculio (Coleoptera: Curculionidae) in Pennsylvania. Environmental Entomology 31: 1240-1250.
43. Loya, L.J. and Hower, A.A. 2003. Infectivity and reproductive potential of the Oswego strain of Heterorhabditis bacteriophora associated with life stages of the clover root curculio, Sitona hispidulus. Journal of Invertebrate Pathology 83: 63-72.
Codling moth, Cydia pomonella
44. Cossentine, J.E., Jensen, L.B. and Moyls, L. 2002. Fruit bins washed with Steinernema carpocapsae (Rhabditida: Steinernematidae) to control Cydia pomonella (Lepidoptera: Tortricidae). Biocontrol Science and Technology 12: 251-258.
45. de Waal, J.Y., Malan, A.P. and Addison, M.F. 2011. Evaluating mulches together with Heterorhabditis zealandica (Rhabditida: Heterorhabditidae) for the control of diapausing codling moth larvae, Cydia pomonella (L.) (Lepidoptera: Tortricidae). Biocontrol Science and Technology 21: 255-270.
46. de Waal, J.Y., Malan, A.P., Levings, J. and Addison, M.F. 2010. Key elements in the successful control of diapausing codling moth, Cydia pomonella (Lepidoptera: Tortricidae) in wooden fruit bins with a South African isolate of Heterorhabditis zealandica (Rhabditida: Heterorhabditidae). Biocontrol Science and Technology. 20: 489-502.
47. Lacey, L.A. and Chauvin, R.L. 1999. Entomopathogenic nematodes for control of diapausing codling moth (Lepidoptera: Tortricidae) in fruit bins. Journal of Economic Entomology 92: 104-109.
48. Lacey, L.A., and Unruh, T.R. 1998. Entomopathogenic nematodes for control of codling moth, Cydia pomonella (Lepidoptera: Tortricidae): Effect of nematode species, concentration, temperature, and humidity. Biological Control 13: 190-197.
49. Lacey, L.A., Arthurs, S.P., Unruh, T.R., Headrick, H. and Fritts, R. 2006. Entomopathogenic nematodes for control of codling moth (Lepidoptera: Tortricidae) in apple and pear orchards: Effect of nematode species and seasonal temperatures, adjuvants, application equipment, and post-application irrigation. Biological Control 37: 214-223.
50. Lacey, L.A., Granatstein, D., Arthurs, S.P., Headrick, H. and Fritts, R. 2006. Use of entomopathogenic nematodes (Steinernematidae) in conjunction with mulches for control of overwintering codling moth (Lepidoptera: Tortricidae). Journal of Entomological Science 41: 107-119.
51. Lacey, L.A., Neven, L.G., Headrick, H.L. and Fritts, R. 2005. Factors affecting entomopathogenic nematodes (Steinerneniatidae) for control of overwintering codling moth (Lepidoptera: Tortricidae) in fruit bins. Journal of Economic Entomology 98: 1863-1869.
52. Lacey, L.A., Shapiro-Ilan, D.I. and Glenn, G.M. 2010. Post-application of anti-desiccant agents improves efficacy of entomopathogenic nematodes in formulated host cadavers or aqueous suspension against diapausing codling moth larvae (Lepidoptera: Tortricidae). Biocontrol Science and Technology. 20: 909-921.
53. Mracek, Z., Becvar, S., Kindlmann, P. and Webster, J.M. 1998. Infectivity and specificity of Canadian and Czech isolates of Steinernema kraussei (Steiner, 1923) to some insect pests at low temperatures in the laboratory. Nematologica 44: 437-448.
54. Navaneethan, T., Strauch, O., Besse, S., Bonhomme, A. and Ehlers, R.U. 2010. Influence of humidity and a surfactant-polymer-formulation on the control potential of the entomopathogenic nematode Steinernema feltiae against diapausing codling moth larvae (Cydia pomonella L.) (Lepidoptera: Tortricidae). Biocontrol 55: 777-788.
55. Unruh, T.R., and Lacey, L.A. 2001. Control of codling moth, Cydia pomonella (Lepidoptera: Tortricidae), with Steinernema carpocapsae: Effects of supplemental wetting and pupation site on infection rate. Biological Control 20: 48-56.
56. Vega, F.E., Lacey, L.A., Reid, A.P., Herard, F., Pilarska, D., Danova, E., Tomov, R. and Kaya, H.K. 2000. Infectivity of a Bulgarian and an American strain of Steinernema carpocapsae against codling moth. Biocontrol 45: 337-343.
Crane flies, Tipula paludosa
57. Oestergaard, J., Belau, C., Strauch, O., Ester, A., van Rozen, K. and Ehlers, R.U. 2006. Biological control of Tipula paludosa (Diptera: Nematocera) using entomopathogenic nematodes (Steinernema spp.) and Bacillus thuringiensis subsp israelensis. Biological Control 39: 525-531.
58. Simard, L., Belair, G., Gosselin, M.E. and Dionne, J. 2006. Virulence of entomopathogenic nematodes (Rhabditida: Steinernematidae, Heterorhabditidae) against Tipula paludosa (Diptera: Tipulidae), a turfgrass pest on golf courses. Biocontrol Science and Technology 16: 789-801.
Cucurbit beetle, Diabrotica speciosa
59. Santos, V., Moino, A., Andalo, V., Moreira, C.C. and de Olinda, R.A. 2011. Virulence of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) for the control of Diabrotica speciosa Germar (Coleoptera: Chrysomelidae). Ciencia e Agrotecnologia 35: 1149-1156.
Cutworms, Agrotis ipsilon and A. segetum
60. Ebssa, L. and Koppenhofer, A.M. 2011. Efficacy and persistence of entomopathogenic nematodes for black cutworm control in turfgrass. Biocontrol Science and Technology 21: 779-796.
61. Kunkel, B.A., Grewal, P.S. and Quigley, M.F. 2004. A mechanism of acquired resistance against an entomopathogenic nematode by Agrotis ipsilon feeding on perennial ryegrass harboring a fungal endophyte. Biological Control 29: 100-108.
62. Richmond, D.S., and Bigelow, C.A. 2009. Variation in endophyte-plant associations influence Black Cutworm (Lepidoptera: Noctuidae) performance and susceptibility to the parasitic nematode Steinernema carpocapsae. Environmental Entomology 38: 996-1004.
63. Shamseldean, M.M., Ibrahim, A.A., Zohdi, N., Shairra, S.A. and Ayaad, T.H. 2008. Effect of Egyptian entomopathogenic nematode isolates on some economic insect pests. Egyptian Journal of Biological Pest Control 18: 81-89.
64. Shapiro-Ilan, D.I., Mbata, G.N., Nguyen, K.B. Peat, S.M., Blackburn, D. and Adams, B.J. 2009. Characterization of biocontrol traits in the entomopathogenic nematode Heterorhabditis georgiana (Kesha strain), and phylogenetic analysis of the nematode’s symbiotic bacteria. Biological Control. 51: 377-387.
65. Shapiro-Ilan, D.I., Stuart, R.J. and McCoy, C.W. 2005. Characterization of biological control traits in the entomopathogenic nematode Heterorhabditis mexicana (MX4 strain). Biological Control 32: 97-103.
Diamondback moth, Plutella xylostella
66. Baur M.E., Kaya H.K., Gaugler R. and Tabashnik, B.E. 1997. Effects of adjuvants on entomopathogenic nematode persistence and efficacy against Plutella xylostella, Biocontrol Science and Technology 7: 513–525.
67. Park, H.W., Kim, H.H., Youn, S.H., Shin, T.S., Bilgrami, A.L., Cho, M.R. and Shin, C.S. 2012. Biological control potentials of insect-parasitic nematode Rhabditis blumi (Nematoda: Rhabditida) for major cruciferous vegetable insect pests. Applied Entomology and Zoology 47: 389-397.
68. Schroer S. and Ehlers R.U. 2005. Foliar application of the entomopathogenic nematode, Steinernema carpocapsae for biological control of diamond black moth larvae (Plutella xylostella). Biological Control 33: 81–86.
69. Schroer S., Sulistyanto D. and Ehlers R.U. 2005. Control of Plutella xylostella using polymerformulated Steinernema carpocapsae and Bacillus thuringiensis in cabbage fields. Journal of Applied Nematology 129:198–204.
70. Schroer S., Ziermann D., Ehlers R.U. 2005. Mode of action of a surfactant-polymer formulation to support performance of the entomopathogenic nematode Steinernema carpocapsae for control of diamondback moth larvae (Plutella xylostella). Biocontrol Science and Technology 15:601–613.
Egyptian cotton leaf worm, Spodoptera littoralis
71. Campos-Herrera, R. and Gutierrez, C. 2008. Comparative study of entomopathogenic nematode isolation using Galleria mellonella (Pyralidae) and Spodoptera littoralis (Noctuidae) as baits. Biocontrol Science and Technology 18: 629-634.
72. Hassan, H.A. and Ibrahim, S.A.M. 2010. Immune response of the cotton leaf worm Spodoptera littoralis (Biosd.) towards entomopathogenic nematodes. Egyptian Journal of Biological Pest Control 20: 45-53.
73. Ibrahim, A.A. and Shairra, S.A. 2011. Effect of eicosanoid biosynthesis inhibitors on the immune response of the Cotton Leaf Worm, Spodoptera littoralis (Boisd.) infected with the nematode, Steinernema glaseri (Rhabditida: Steinernematidae). Egyptian Journal of Biological Pest Control 21: 197-202.
Fall webworms, Hyphantria cunea
74. Chkhubianishvili, T., Mikaia, N., Malania, I. and Kakhadze, M. 2007. Susceptibility of entomopathogenic nematodes to the fall webworm Hyphantria cunea Drury (Lepidoptera: Arctiidae). Bulletin of the Georgian National Academy of Sciences 175: N2.
Filbertworm, Cydia latiferreana
75. Bruck, D.J. and Walton, V.M. 2007. Susceptibility of the filbertworm (Cydia latiferreana, Lepidoptera: Tortricidae) and filbert weevil (Curculio occidentalis, Coleoptera: Curculionidae) to entomopathogenic nematodes. Journal of Invertebrate Pathology. 96: 93–96.
76. Chambers, U. Bruck, D.J., Olsen, J. and Walton, V.M. 2010. Control of overwintering filbertworm (Lepidoptera: Tortricidae) larvae with Steinernema carpocapsae. Journal of Economic Entomology. 103: 416-422.
Flea beetles, Phyllotreta spp.
77. Morris, O.N. 1987. Evaluation of the nematode, Steinernema-feltiae filipjev, for the control of the crucifer flea beetle, phyllotreta-cruciferae (goeze) (coleoptera, chrysomelidae). Canadian Entomologist 119: 95-101.
78. Trdan, S., Vidrih, M., Valic, N. and Laznik, Z. 2008. Impact of entomopathogenic nematodes on adults of Phyllotreta spp. (Coleoptera: Chrysomelidae) under laboratory conditions. Acta Agriculturae Scandinavica Section B-Soil and Plant Science 58: 169-175.
79. Xu, C.X., De Clercq, P., Moens, M., Chen, S.L and Han, R.C. 2010. Efficacy of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) against the striped flea beetle, Phyllotreta striolata. Biocontrol 55: 789-797.
Fungus gnats, Bradysia coprophila
80. Harris, M.A., Oetting, R.D., Gardner, W.A., 1995. Use of entomopathogenic nematodes and new monitoring technique for control of fungus gnats, Bradysia coprophila (Diptera: Sciaridae), in floriculture. Biological Control 5, 412-418.
81. Jagdale, G. B., Casey, M. L., Grewal, P. S. and Lindquist, R. K. 2004. Application rate and timing, potting medium and host plant on the efficacy of Steinernema feltiae against the fungus gnat, Bradysia coprophila, in floriculture. Biological Control 29: 296-305.
82. Jagdale, G. B., Casey, M. L., Grewal, P. S. and Luis Cañas. 2007. Effect of entomopathogenic nematode species, split application and potting medium on the control of the fungus gnat, Bradysia difformis (Diptera: Sciaridae), in the greenhouse at alternating cold and warm temperatures. Biological Control 43: 23-30.
83. Kim, H.H., Choo, H.Y., Kaya, H.K., Lee, D.W., Lee, S.M., Jeon, H.Y., 2004. Steinernema carpocapsae (Rhabditida: Steinernematidae) as a biological control agent against the fungus gnat Bradysia agrestis (Diptera: Sciaridae) in propogation houses. Biocontrol Science and Technology 14, 171-183.
84. Lindquist R., Piatkowski J. 1993. Evaluation of entomopathogenic nematodes for control of fungus gnat larvae. Bull. Int. Organiz. Biol. Integr. Control Noxious Animals and Plants. 16: 97-100.
House flies, Musca domestica
85. Renn, N. 1995. Mortality of immature houseflies (Musca-domestica l) in artificial diet and chicken manure after exposure to encapsulated entomopathogenic nematodes (Rhabditida, Steinernematidae, heterorhabditidae). Biocontrol Science and Technology 5: 349-359.
86. Renn, N. 1998. Routes of penetration of the entomopathogenic nematode Steinernema feltiae attacking larval and adult houseflies (Musca domestica). Journal of Invertebrate Pathology 72: 281-287.
87. Renn, N. 1998. The efficacy of entomopathogenic nematodes for controlling housefly infestations of intensive pig units. Medical and Veterinary Entomology 12: 46-51.
88. Renn, N. and Wright, E. 2000. The effect of artificial substrates on the pathogenicity of Steinernema feltiae (Rhabditida: Steinernematidae) to adult Musca domestica (Diptera: Muscidae). Nematology 2: 217-222.
89. Taylor, D.B., Szalanski, A.L., Adams, B.J. and Peterson, R.D. 1998. Susceptibility of house fly (Diptera: Muscidae) larvae to entomopathogenic nematodes (Rhabditida: Heterorhabditidae, Steinernematidae). Environmental Entomology 27: 1514-1519.
Japanese beetle, Popillia japonica
90. Alm, S.R., Yeh, T., Hanula, J.L. and Georgis, R. 1992. Biological control of Japanese, oriental and black turfgrass ataenius beetel (Coleoptera, Scarabidae) larvae with entomopathogenic nematodes (Nematoda, Steinernematidae, Heterorhabditidae). Journal of Economic Entomology. 85: 1660-1665.
91. Grewal, P.S., Koppenhofer, A.M., and Choo, H.Y., 2005. Lawn, turfgrass and Pasture applications. In: Nematodes As Biocontrol Agents. Grewal, P.S. Ehlers, R.-U., Shapiro-Ilan, D. (eds.). CAB publishing, CAB International, Oxon. Pp 147-166.
92. Hao, Y.J., Montiel, R., Lucena, M.A., Costa, M. and Simoes, N. 2012. Genetic diversity and comparative analysis of gene expression between Heterorhabditis bacteriophora Az29 and Az36 isolates: Uncovering candidate genes involved in insect pathogenicity. Experimental Parasitology 130: 116-125.
93. Koppenhofer, A.M., Fuzy, E.M., Crocker, R.L., Gelernter, W.D. and Polavarapu, S. 2004. Pathogenicity of Heterorhabditis bacteriophora, Steinernema glaseri, and S. scarabaei (Rhabditida: Heterorhabditidae, Steinernematidae) against 12 white grub species (Coleoptera: Scarabaeidae). Biocontrol Science and Technology. 14: 87-92.
94. Koppenhofer, A.M., Grewal, P.S. and Fuzy, E.M. 2006. Virulence of the entomopathogenic nematodes Heterorhabditis bacteriophora, Heterorhabditis zealandica, and Steinernema scarabaei against five white grub species (Coleoptera: Scarabaeidae) of economic importance in turfgrass in North America. Biological Control 38: 397-404.
95. Maneesakorn, P., An, R., Grewal, P.S.and Chandrapatya, A. 2010. Virulence of our new strains of entomopathogenic nematodes from Thailand against second instar larva of the Japanese beetle, Popillia japonica (Coleoptera: Scarabaeidae). Thai Journal of Agricultural Science.43: 61-66.
96. Morales-Rodriguez, A. and Peck, D.C. 2009. Synergies between biological and neonicotinoid insecticides for the curative control of the white grubs Amphimallon majale and Popillia japonica. Biological Control. 51: 169-180.
97. Poinar, G. O., Jr., T. Jackson, and M. Klein. 1987. Heterorhabditis megidis sp. n. (Heterorhabditidae: Rhabditida) parasitic in the Japanese beetle, Popillia japonica (Scarabaeidae: Coleoptera), in Ohio. Proceedings of the Helminthological Society of Washington 54:53-59.
98. Power, K.T., An, R. and Grewal, P.S. 2009. Effectiveness of Heterohabditis bacteriophora strain GPS11 applications targeted against different instars of the Japanese beetle Popillia japonica. Biological Control. 48: 232-236.
99. Yeh, T. and Alm, S.R. 1995. Evaluation of Steinernema glaseri (Nematoda: Steinernematidae) for biological control of Japanese and oriental beetles (Coleoptera, Searabaeidae). Journal of Economic Entomology. 88: 1251-1255.
Leaf minors, Liriomyza trifolii and L. huidobrensis
100. Hara, A.H., Kaya, H.K., Gaugler, R., Lebeck, L.M. and Mello, C.L. 1993. Entomopathogenic nematodes for biological control of the leafminer,
Liriomyza trifolii (Diptera: Agromyzidae). Entomophaga 38, 359-369.
101. Head, J. and Walters, K.F.A. 2003. Augmentation biological control utilising the entomopathogenic nematode,
Steinernema feltiae, against the South American Leafminer,
Liriomyza huidobrensis. Proceedings of the 1st International Symposium on Biological Control, (Hawaii, USA, 13-18 January 2002).
USDA Forest Service, FHTET-03-05, 136-140.
102. Olthof, T.H.A. and Broadbent, A.B. 1992. Evaluation of steinernematid nematodes for control of a leafminer,
Liriomyza trifolii, in greenhouse chrysanthemums. Journal of Nematology 24, 612.
103. Stever, D.D. and Rice, B. 2004. A Comparison between two species of nematode (
Steinernema carpocapsae and
Steinernema feltiae) applied through a foliar spray to control leafminer (
Liriomyza trifolii) in
Dendranthema grandiflora. Hortscience 39: 846-846.
104. Tong-Xian Liu, Le Kang, K.M.Heinz, J.Trumble. 2008. Biological control of
Liriomyza leafminers: progress and perspective. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources, 2009, 4, No. 004, 16 pp.
105. Williams, E.C. and MacDonald, O.C., 1995. Critical factors required by the nematode
Steinernema feltiae for the control of the leafminers
Liriomyza huidobrensis,
Liriomyza bryoniae and
Chromatomyia syngenesiae. Annals of Applied Biology. 127, 329-341.
106. Williams, E.C. and Walters, K.F.A. 1994. Nematode control of leafminers: Efficacy, temperature and timing. Brighton Crop Protection Conference – Pests and Disease. 1079-1084.
107. Williams, E.C. and Walters, K.F.A. 2000. Foliar application of the entomopathogenic nematode
Steinernema feltiae against leafminers on vegetables. Biocontrol Science and Technology 10, 61-70.
Leopard moth, Zeuzera pyrina
108. Abdel-Kawy, A.G.M., El-Bishry, M.H. & El-Kifi, T.A.H. 1992: Controlling the leopard moth borer,
Zeuzera pyrina by three entomopathogenic nematode species in the field.
Faculty of Agriculture Bulletin of Univercity of Cairo, 43: 769-780.
Mediterranean fruit fly Ceratitis capitata
109. Bazman, I., Ozer, N. and Hazir, S. 2008. Bionomics of the entomopathogenic nematode,
Steinernema weiseri (Rhabditida: Steinernematidae). Nematology 10: 735-742.
110. Campos-Herrera, R. and Gutierrez, C. 2009. Screening Spanish isolates of steinernematid nematodes for use as biological control agents through laboratory and greenhouse microcosm studies. Journal of Invertebrate Pathology. 100: 100-105.
111. Karagoz, M., Gulcu, B., Hazir, C., Kaya, H.K. and Hazir, S. 2009. Biological control potential of Turkish entomopathogenic nematodes against the Mediterranean fruit fly
Ceratitis capitata. Phytoparasitica. 37: 153-159.
112.
Malan, A.P. and Manrakhan, A. 2009. Susceptibility of the Mediterranean fruit fly (Ceratitis capitata) and the natal fruit fly (Ceratitis rosa) to entomopathogenic nematodes. Journal of Invertebrate Pathology. 100: 47-49.
113. Raja, R.K., Sivaramakrishnan, S. and Hazir, S. 2011. Ecological characterisation of
Steinernema siamkayai (Rhabditida: Steinernematidae), a warm-adapted entomopathogenic nematode isolate from India. Biocontrol 56: 789-798.
114. Rohde, C., Moino, A., da Silva, M.A.T., Carvalho, F.D.and Ferreira, C.S. 2010. Influence of soil temperature and moisture on the infectivity of entomopathogenic nematodes (Rhabditida: Heterorhabditidae, Steinemematidae) against larvae of
Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). NeotropicaL Entomology. 39: 608-611.
115. Soliman, N. A. 2007. Efficacy of the entomopathogenic nematodes,
Steinernema riobravis Cabanillas and
Heterorhabditis bacteriophora (native strain) against the peach fruit fly, Bactrocera zonata (Saunders) and the Mediterranean fruit fly, Ceratitis capitata (Wiedemann). Egyptian Journal of Biological Pest control. 17: 77-82.
116. Soliman, N.A. 2007. Pathogenicity of three entomopathogenic nematodes to the Peach fruit fly,
Bacterocera zonata (Saunders) and the Mediterranean fruit fly,
Ceratitis capitata (Wiedemann) (Diptera : Tephritidae). Egyptian Journal of Biological Pest Control 17: 121-124.
Mole crickets, Scapteriscus vicinus
117. Adjei, M.B., Smart, Jr. G.C., Frank, J.H. and Leppla, N.C. 2006. Control of pest mole crickets (Orthoptera: Gryllotalpidae) on pasture with the nematode
Steinernema scapterisci (Rhabditida: Steinernematidae). Florida Entomologist 89: 532-535.
118. Barbara, K.A. and Buss, E.A. 2004. Survival and infectivity of
Steinernema scapterisci (Nematoda: Steinernematidae) after contact with soil drench solutions. Florida Entomologist 87: 300-305.
119. Barbara, K.A. and Buss, E.A. 2005. Integration of insect parasitic nematodes (Rhabditida Steinernematidae) with insecticides for control of pest mole crickets (Orthoptera: Gryllotalpidae: Scapteriscus spp.). Journal of Economic Entomology 98: 689-693.
120. Barbara, K.A. and Buss, E.A. 2006. Augmentative applications of
Steinernema scapterisci (Nematoda: Steinernematidae) for mole cricket (Orthoptera: Gryllotalpidae) control on golf courses. Florida Entomologist 89: 257-262.
121. Frank, J.H. 1999. Beneficial nematode killing mole crickets in Florida pastures, still spreading. Florida Turf Digest 16: 32, 34-35.
122. Frank, J.H., Grissom, C., Williams, C., Jennings. E., Lippi, C. and Zerba, E. 1999. A beneficial nematode is killing pest mole crickets in some Florida pastures and is spreading. Florida Cattleman 63: 31-32.
123. Georgis, R., Poinar Jr., G.O., 1994. Nematodes as biopesticides in turf and ornamentals. In: Leslie, A. (Ed.), Integrated Pest Management for Turf and Ornamentals. CRC Press, Boca Raton, FL, USA, pp. 477–489.
124. Nguyen, K.B. and Smart, G.C. 1990.
Steinernema scapterisci n. sp. (Rhabditida: Steinernematidae). Journal of Nematology 22: 187-199.
125. Nguyen, K.B. and Smart, G.C. 1991. Mode of entry and site of development of
Steinernema scapterisci in mole crickets. Journal of Nematology 23: 267-268.
126. Nguyen, K.B. and Smart, G.C. 1991. Pathogenicity of
Steinernema scapterisci to selected invertebrates. Journal of Nematology 23: 7-11.
127. Nguyen, K.B. and Smart, G.C. 1991. Vertical distribution of
Steinernema scapterisci. Journal of Nematology 23: 574-578.
128. Nguyen, K.B. and Smart, G.C. 1992. Addendum to the morphology of
Steinernema scapterisci. Journal of Nematology 24: 478-481.
129. Nguyen, K.B. and Smart, G.C. 1992. Life cycle of
Steinernema scapterisci Nguyen & Smart, 1990. Journal of Nematology 24: 160-169.
130. Parkman, J.P., Frank, J.H., Nguyen, K.B. and Smart, G.C. 1993. Dispersal of
Steinernema scapterisci (Rhabditida: Steinernematidae) after inoculative applications for mole cricket (Orthoptera: Gryllotalpidae) control in pastures. Biological Control 3: 226-232.
131.
Parkman, J.P., Frank, J.H., Nguyen, K.B. and Smart, G.C. 1994. Inoculative release of Steinernema scapterisci (Rhabditida: Steinernematidae) to suppress pest mole crickets (Orthoptera: Gryllotalpidae) on golf courses. Environmental Entomology 23: 1331-1337.
Navel orangeworm, Amyelois transitella
132. Hodson, A.K., Siegel, J.P. and Lewis, E.E. 2012. Ecological influence of the entomopathogenic nematode,
Steinernema carpocapsae, on pistachio orchard soil arthropods. Pedobiologia 55: 51-58.
Peach borer, Synanthedon exitiosa
133.
Cottrell, T.E. and Shapiro-Ilan, D.I. 2006. Susceptibility of the peachtree borer, Synanthedon exitiosa, to Steinernema carpocapsae and Steinernema riobrave in laboratory and field trials. Journal of Invertebrate Pathology 92: 85-88.
134. Shapiro-Ilan, D.I., Cottrell, T.E., Mizell, R.F., Horton, D.L. and Davis, J. 2009. A novel approach to biological control with entomopathogenic nematodes: prophylactic control of the peachtree borer,
Synanthedon exitiosa. Biological Control. 48: 259-263.
Pecan weevil, Curculio caryae and C. hicoriae
135. Shapiro-Ilan, D. and Gardner, W.A. 2012. Improved control of
Curculio caryae (Coleoptera: Curculionidae) through multi-stage pre-emergence applications of
Steinernema carpocapsae. Journal of Entomological Science 47: 27-34.
136. Shapiro-Ilan, D. and Hall, M.J. 2012. Susceptibility of adult nut Curculio,
Curculio hicoriae (Coleoptera: Curculionidae) to entomopathogenic nematodes under laboratory conditions. Journal of Entomological Science 47: 375-378.
137. Shapiro-Ilan, D.I., Cottrell, T.E. and Wood, B.W. 2011. Effects of combining microbial and chemical insecticides on mortality of the Pecan weevil (Coleoptera: Curculionidae). Journal of Economic Entomology 104: 14-20.
138.
Shapiro-Ilan, D.I., Cottrell, T.E., Brown, I., Gardner, W.A., Hubbard, R.K. and Wood, B.W. 2006. Effect of soil moisture and a surfactant on entomopathogenic nematode suppression of the pecan weevil, Curculio caryae. Journal of Nematology 38: 474-482.
139. Shapiro-Ilan, D.I., Cottrell, T.E., Gardner, W.A., Leland, J. and Behles, R.W. 2009. Laboratory mortality and mycosis of adult
Curculio caryae (Coleoptera: Curculionidae) following application of
Metarhizium anisopliae in the laboratory or field. Journal of Entomological Science. 44: 24-36.
140. Shapiro-Ilan, D.I., Dutcher, J.D. and Hatab, M. 2005. Recycling potential and fitness of steinernematid nematodes cultured in
Curculio caryae and
Gaileria mellonella. Journal of Nematology 37: 12-17.
141.
Shapiro-Ilan, D.I., Jackson, M., Reilly, C.C. and Hotchkiss, M.W. 2004. Effects of combining an entomopathogenic fungi or bacterium with entomopathogenic nematodes on mortality of Curculio caryae (Coleoptera: Curculionidae). Biological Control 30: 119-126.
142. Shapiro-Ilan, D.I., Mizell, R.F., Cottrell, T.E. and Horton, D.L. 2008. Control of plum curculio,
Conotrachelus nenuphar, with entomopathogenic nematodes: Effects of application timing, alternate host plant, and nematode strain. Biological Control 44: 207-215.
143. Shapiro-Ilan, D.I., Stuart, R.J. and McCoy, C.W. 2005. Characterization of biological control traits in the entomopathogenic nematode
Heterorhabditis mexicana (MX4 strain). Biological Control 32: 97-103.
Pine weevil, Hylobius abietis
144. Dillon, A.B., Moore, C.P., Downes, M.J. and Griffin, C.T. 2008. Evict or infect? Managing populations of the large pine weevil,
Hylobius abietis, using a bottom-up and top-down approach. Forest Ecology and Management 255: 2634-2642.
145. Ennis, D.E., Dillon, A.B. and Griffin, C.T. 2010. Pine weevils modulate defensive behaviour in response to parasites of differing virulence. Animal Behaviour 80: 283-288.
146.
Everard, A., Griffin, C.T. and Dillon, A.B. 2009. Competition and intraguild predation between the braconid parasitoid Bracon hylobii and the entomopathogenic nematode Heterorhabditis downesi, natural enemies of the large pine weevil, Hylobius abietis. Bulletin of Entomological Research. 99: 151-161.
147. Girling, R.D., Ennis, D., Dillon, A.B. and Griffin, C.T. 2010. The lethal and sub-lethal consequences of entomopathogenic nematode infestation and exposure for adult pine weevils,
Hylobius abietis (Coleoptera: Curculionidae). Journal of Invertebrate Pathology 104: 195-202.
148. Kruitbos, L.M., Heritage, S. and Wilson, M.J. 2009. Phoretic dispersal of entomopathogenic nematodes by
Hylobius abietis. Nematology. 11: 419-427.
Plum weevil, Conotrachelus nenuphar
149. Alston, D.G., Rangel, D.E.N., Lacey, L.A., Golez, H.G., Kim, J.J. and Roberts, D.W. 2005. Evaluation of novel fungal and nematode isolates for control of
Conotrachelus nenuphar (Coleoptera: Curculionidae) larvae. Biological Control 35: 163-171.
150. Kim, H.G. and Alston, D.G. 2008. Potential of two entomopathogenic nematodes for suppression of plum Curculio (
Conotrachelus nenuphar, Coleoptera: Curculionidae) life stages in northern climates. Environmental Entomology 37: 1272-1279.
151. Pereault, R.J., Whalon, M.E. and Alston, D.G. 2009. Field efficacy of entomopathogenic fungi and nematodes targeting caged last-instar plum Curculio (Coleoptera: Curculionidae) in Michigan cherry and apple Orchards. Environmental Entomology. 38: 1126-1134.
152.
Shapiro-Ilan, D.I., Mizell, R.F. and Campbell, J.F. 2002. Susceptibility of the plum curculio, Conotrachelus nenuphar to entomopathogenic nematodes. Journal of Nematology 34: 246-249.
153. Shapiro-Ilan, D.I., Mizell, R.F., Cottrell, T.E and Horton, D.L. 2004. Measuring field efficacy of
Steinernema feltiae and
Steinernema riobrave for suppression of plum curculio,
Conotrachelus nenuphar larvae. Biological Control 30: 496–503.
154. Shapiro-Ilan, D.I., Mizell, R.F., Cottrell, T.E., Horton, D.L. 2008. Control of plum curculio,
Conotrachelus nenuphar with entomopathogenic nematodes: Effects of application timing, alternate host plant, and nematode strain. Biological Control. 44: 207-215.
Shore flies, Scatella stagnalis
155. Lindquist, R., Buxton, J. and Piatkowski, J. 1994. Biological control of sciarid flies and shore flies in glasshouses. Brighton Crop Protection Conference, Pests and Diseases, BCPC Publications 3, 1067-1072.
156. Morton, A. and Garcia del Pino, F. 2003. Potential of entomopathogenic nematodes for the control of shore flies (Scatella stagnalis). Growing Biocontrol Markets Challenge Research and Development. 9th European Meeting IOBC/WPRS Working Group “Insect Pathogens and Entomopathogenic Nematodes”, Abstracts, 67.
157. Morton, A., Garcia del Pino, F., 2007. Susceptibility of shore fly Scatella stagnalis to five entomopathogenic nematode strains in bioassays. Biocontrol 52: 533-545.
158. Vanninen, I., Koskula, H. 2000. Biological control of the shore fly (Scatella tenuicosta) with steinernematid nematodes and Bacillus thuringiensis var. thuringiensis in peat and rockwool. Biocontrol Science and Technology 13: 47-63.
Sod webworm, Herpetogramma phaeopteralis
159. Nastaran Tofangsazie, N., Arthurs, S.P. and Cherry, R. https://edis.ifas.ufl.edu/in968
Spruce webworm, Cephalcia abietis
160. Fischer, P. 1996. The entomoparasitic nematode species Steinernema feltiae (Nematoda: Steinernematidae) as a mortality factor of the spruce webworm Cephalcia abietis (Hymenoptera: Pamphiliidae). Entomologia Generalis 21: 107-115.
Stable fly, Stomoxys calcitrans
161. Pierce, L. 2008. Efficacy of entomopathogenic nematodes used for control of stable flies (Stomoxys calcitrans) at round bale feeding sites in pastures. Abstract, Annual Meeting of Entomological Society of America.
Stored grain pests
162. Athanassiou, C.G., Kavallieratos, N.C., Menti, H. and Karanastasi, E. 2010. Mortality of four stored product pests in stored wheat when exposed to doses of three entomopathogenic nematodes. Journal of Economic Entomology 103: 977-984.
163. Athanassiou, C.G., Palyvos, N.E. and Kakoull-Duarte, T. 2008. Insecticidal effect of Steinernema feltiae (Filipjev) (Nematoda: Steinernematidae) against Tribolium confusum du Val (Coleoptera: Tenebrionidae) and Ephestia kuehniella (Zeller) (Lepidoptera: Pyralidae) in stored wheat. Journal of Stored Products Research. 44: 52-57.
164. Fayyaz S. and Javed, S. 2009. Laboratory evaluation of seven Pakistani strains of entomopathogenic nematodes against a stored grain insect pest, pulse beetle Callosobruchus chinensis (L.). Journal of Nematology 41: 255-260.
165. Mbata, G.N. and Shapiro-Ilan, D.I. 2005. Laboratory evaluation of virulence of heterorhabditid nematodes to Plodia interpunctella Hübner (Lepidoptera: Pyralidae). Environmental Entomology 34: 676 – 682.
166. Ramos-Rodríguez, O., Campbell, J. F. and Ramaswamy, S. 2007. Efficacy of the entomopathogenic nematodes Steinernema riborave against the stored-product pests Tribolium castaneum and Plodia interpunctella. Biological Control 40:15 -21.
167. Ramos-Rodriguez, O., Campbell, J.F. and Ramaswamy, S.B. 2006. Pathogenicity of three species of entomopathogenic nematodes to some major stored-product insect pests. Journal of Stored Products Research 42: 241-252.
168. Tradan, S., Vidric, M., and Valic, N. 2006. Activity of four entomopathogenic nematodes against young adult of Sitophilus granarious (Coleptera: Curculionidae) and Oryzophilus surinamensis (Coleoptera: Silvanidae) under laboratory condition. Plant Disease and Protection. 113: 168 – 173.
Strawberry root borer, Nemocestes incomptus
169. Georgis, R and Poinar, GO. 1984. Field control of the strawberry root weevil, Nemocestes-incomptus, by Neoaplectanid nematodes (Steinernematidae, Nematoda). Journal of Invertebrate Pathology 43: 130-131.
Strawberry root weevil, Otiorhynchus ovatus and Ptiorhynchus ovatus
170.
Berry, R.E., Liu, J. and Groth, E. 1997. Efficacy and persistence of Heterorhabditis marelatus (Rhabditida: Heterorhabditidae) against root weevils (Coleoptera: Curculionidae) in strawberry. Environmental Entomology 26: 465-470.
171. Booth, S.R., Tanigoshi, L.K. and Shanks, C.H. 2002. Evaluation of entomopathogenic nematodes to manage root weevil larvae in Washington state cranberry, strawberry, and red raspberry. Environmental Entomology 31: 895-902.
172.
Simser, D. and Roberts, S. 1994. Suppression of strawberry root weevil, Otiorhynchus-ovatus, in cranberries by entomopathogenic nematodes (Nematoda, Steinernematidae and Heterorhabditidae. Nematologica 40: 456-462.
173.
Vainio, A. and Hokkanen, H.M.T. 1993. The potential of entomopathogenic fungi and nematodes against Otiorhynchus-ovatus L and O. dubius strom (Col, Curculionidae) in the field. Journal of Applied Entomology-Zeitschrift fur Angewandte Entomologie. 115: 379-387.
Strawberry crown moth, Synanthedon bibionipennis
174.
Bruck, D.J., Edwards, D.L. and Donahue, K.M. 2008. Susceptibility of the strawberry crown moth (Lepidoptera: Sesiidae) to entomopathogenic nematodes. Journal of Economic Entomology 101: 251-255.
Tick, Rhipicephalus (Boophilus) microplus
175. de Carvalho, L.B., Furlong, J., Prata, M.C.D., dos Reis, E.S., Batista, E.S.D., Faza, A.P. and Leite R.C. 2010. Evaluation in vitro of the infection times of engorged females of
Rhipicephalus (Boophilus) microplus by the entomopathogenic nematode
Steinernema glaseri CCA strain. Ciencia Rural. 40: 939-943.
176.
Freitas-Ribeiro G.M., Furlong, J., Vasconcelos, V.O., Dolinski, C. and Loures-Ribeiro, A. 2005. Analysis of biological parameters of Boophilus microplus Canestrini, 1887 exposed to entomopathogenic nematodes Steinernema carpocapsae Santa Rosa and all strains (Steinernema: Rhabditida). Brazilian Archives of Biology and Technology. 48: 911-919.
177. Kocan, K.M., Pidherney, M.S., Blouin, E.F., Claypool, P.L., Samish, M. and Glazer, I. 1998. Interaction of entomopathogenic nematodes (Steinernematidae) with selected species of ixodid ticks (Acari: Ixodidae). Journal of Medical Entomology. 35: 514-520.
178. Monteiro, C.M.D., Prata, M.C.D., Furlong, J., Faza, A.P., Mendes, A.S., Andalo, V. and Moino, A.2010.
Heterorhabditis amazonensis (Rhabditidae: Heterorhabditidae), strain RSC-5, for biological control of the cattle tick
Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Parasitology Research. 106: 821-826.
179.
Reis-Menini, C.M.R., Prata, M.C.A., Furlong, J. and Silva, E.R. 2008. Compatibility between the entomopathogenic nematode Steinernema glaseri (Rhabditida: Steinernematidae) and an acaricide in the control of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Parasitology Research. 103: 1391-1396.
Western flower thrips, Frankliniella occidentalis
180. Arthurs, S. and Heinz, K.M. 2006. Evaluation of the nematodes
Steinernema feltiae and
Thripinema nicklewoodi as biological control agents of western flower thrips
Frankliniella occidentalis infesting chrysanthemum. Biocontrol Science and Technology 16: 141-155.
181. Ebssa, L., Borgemeister, C. and Poehling, H.M. 2004. Effects of post-application irrigation and substrate moisture on the efficacy of entomopathogenic nematodes against western flower thrips,
Frankliniella occidentalis. Entomologia Experimentalis et Applicata 112: 65-72.
182.
Ebssa, L., Borgemeister, C. and Poehling, H.M. 2006. Simultaneous application of entomopathogenic nematodes and predatory mites to control western flower thrips Frankliniella occidentalis. Biological Control 39: 66-74.
183. Ebssa, L., Borgemeister, C., Berndt, O. and Poehling, H.M. 2001. Efficacy of entomopathogenic nematodes against soil-dwelling life stages of western flower thrips,
Frankliniella occidentalis (Thysanoptera: Thripidae). Journal of Invertebrate Pathology 78: 119-127.
184. North, J.P., Cuthbertson, A.G.S. and Walters, K.F.A. 2006. The efficacy of two entomopathogenic biocontrol agents against adult
Thrips palmi (Thysanoptera: Thripidae). Journal of Invertebrate Pathology 92: 89-92.
185. Premachandra, D.W.T.S., Borgemeister, C., Berndt, O., Ehlers, R.U. and Poehling, H.M. 2003. Laboratory bioassays of virulence of entomopathogenic nematodes against soil-inhabiting stages of
Frankliniella occidentalis Pergande (Thysanoptera: Thripidae). Nematology 5: 539-547.
186. Trdan, S., Znidarcic, D. and Vidrih, M. 2007. Control of
Frankliniella occidentalis on glasshouse-grown cucumbers: an efficacy comparison of foliar application of
Steinernema feltiae and spraying with abamectin. Russian Journal of Nematology 15: 25-34.
Western corn rootworm Diabrotica virgifera virgifera
187.
Hiltpold, I., Hibbard, B.E., French, B.W. and Turlings, T.C.J. 2012. Capsules containing entomopathogenic nematodes as a Trojan horse approach to control the western corn rootworm. Plant and Soil 358: 10-24.
188. Petzold-Maxwell, J.L., Cibils-Stewart, X., French, B.W. and Gassmann, A.J. 2012. Adaptation by western corn rootworm (Coleoptera: Chrysomelidae) to Bt Maize: inheritance, fitness costs, and feeding preference. Journal of Economic Entomology 105: 1407-1418.
189. Petzold-Maxwell, J.L., Jaronski, T. and Gassmann, A.J. 2012. Tritrophic interactions among Bt maize, an insect pest and entomopathogens: effects on development and survival of western corn rootworm. Annals of Applied Biology 160: 43-55.
Whiteflies, Bemisia tabaci and Trialeurodes vaporariorum
190. Cuthbertson, A.G.S., Blackburn, L.F., Eyre, D.P., Cannon, R.J.C., Miller, J. and Northing, P. 2011. Bemisia tabaci: The current situation in the UK and the prospect of developing strategies for eradication using entomopathogens. Insect Science 18: 1-10.
191. Cuthbertson, A.G.S., Mathers, J.J., Northing, P., Prickett, A.J. and Walters, K.F.A. 2008. The integrated use of chemical insecticides and the entomopathogenic nematode,
Steinernema carpocapsae (Nematoda: Steinernematidae), for the control of sweetpotato whitefly,
Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Science 15: 447-453.
192. Head J., Lawrence A.J. and Walters K.F.A. 2004. Efficacy of the entomopathogenic nematode,
Steinernema feltiae against
Bemisia tabaci in relation to plant species, Journal of Applied Entomology. 128:543–547.
193. Laznik, Z., Znidarcic, D. and Trdan, S. 2011. Control of Trialeurodes vaporariorum (Westwood) adults on glasshouse-grown cucumbers in four different growth substrates: an efficacy comparison of foliar application of Steinernema feltiae (Filipjev) and spraying with thiamethoxamn. Turkish Journal of Agriculture and Forestry 35: 631-640.
194. Shrestha, Y.K. and Lee, K.Y. 2012. Oral toxicity of
Photorhabdus culture media on gene expression of the adult sweetpotato whitefly,
Bemisia tabaci. Journal of Invertebrate Pathology 109: 91-96.
White grubs (Summer Chafer), Amphimallon solstitiale
195.
Glare T.R., Jackson T.A., Zimmermann G. 1993. Occurrence of Bacillus popilliae and two nematode pathogens in populations of Amphimallon solstitialis (Col.: Scarabaeidae) near Darmstadt, Germany. Entomophaga. 38: 441-450.
White grubs (Oriental beetle), Anomala orientalis, Exomala orientalis and Blitopertha orientalis
196.
Alm, S.R., Yeh, T., Hanula, J.L. and Georgis, R. 1992. Biological control of Japanese, oriental and black turfgrass ataenius beetel (Coleoptera, Scarabidae) larvae with entomopathogenic nematodes (Nematoda, Steinernematidae, Heterorhabditidae). Journal of Economic Entomology. 85: 1660-1665.
197. Choo, H.Y., Kaya, H.K., Huh, J., Lee, D.W., Kim, H.H., Lee, S.M. and Choo, Y.M. 2002. Entomopathogenic nematodes (
Steinernema spp. and
Heterorhabditis bacteriophora) and a fungus
Beauveria brongniartii for biological control of the white grubs,
Ectinohoplia rufipes and
Exomala orientalis, in Korean golf courses. Biocontrol 47: 177-192.
198.
Grewal, P.S., Grewal, S.K., Malik, V.S. and Klein, M.G. 2007. Differences in susceptibility of introduced and native white grub species to entomopathogenic nematodes from various geographic localities. Biological Control 24: 230-237.
199. Koppenhofer, A.M. and Fuzy E.M. 2004. Effect of white grub developmental stage on susceptibility to entomopathogenic nematodes. Journal of Economic Entomology 97: 1842-1849.
200. Koppenhofer, A.M. and Fuzy, E.M. 2003.
Steinernema scarabaei for the control of white grubs. Biological Control 28: 47-59.
201. Koppenhofer, A.M. and Fuzy, E.M. 2006. Effect of soil type on infectivity and persistence of the entomopathogenic nematodes
Steinernema scarabaei,
Steinernema glaseri,
Heterorhabditis zealandica, and
Heterorhabditis bacteriophora. Journal of Invertebrate Pathology. 92: 11-22.
202. Koppenhofer, A.M. and Fuzy, E.M. 2008. Attraction of four entomopathogenic nematodes to four white grub species. Journal of Invertebrate Pathology 99: 227-234.
203. Koppenhofer, A.M. and Fuzy, E.M. 2008. Earl timing and new combinations to increase the efficacy of neonicotinoid-entomopathogenic nematode (Rhabditida: Heterorhabditidae) combinations against white grubs (Coleoptera: Scarabaeidae. Pest Management Science 64: 725-735.
204. Koppenhofer, A.M. and Fuzy, E.M. 2008c. Effect of the anthranilic diamide insecticide, chlorantraniliprole, on
Heterorhabditis bacteriophora (Rhabditida: Heterorhabditidae) efficacy against white grubs (Coleoptera: Scarabaeldae). Biological Control. 45: 93-102.
205. Koppenhofer, A.M. and Fuzy, E.M. 2009. Long-term effects and persistence of
Steinernema scarabaei applied for suppression of
Anomala orientalis (Coleoptera: Scarabaeidae). Biological Control 48: 63-72.
206. Koppenhofer, A.M., Brown, I.M., Gaugler, R., Grewal, P.S., Kaya, H.K. and Klein M.G. 2000. Synergism of entomopathogenic nematodes and imidacloprid against white grubs: Greenhouse and field evaluation. Biological Control 19: 245-251.
207. Koppenhofer, A.M., Cowles, R.S., Cowles, E.A., Fuzy, E.M. and Baumgartner, L. 2002. Comparison of neonicotinoid insecticides as synergists for entomopathogenic nematodes. Biological Control 24: 90-97.
208. Koppenhofer, A.M., Fuzy, E.M., Crocker, R.L., Gelernter, W.D. and Polavarapu, S. 2004. Pathogenicity of
Heterorhabditis bacteriophora,
Steinernema glaseri, and
S. scarabaei (Rhabditida: Heterorhabditidae, Steinernematidae) against 12 white grub species (Coleoptera: Scarabaeidae). Biocontrol Science and Technology 14: 87-92.
209.
Koppenhofer, A.M., Grewal, P.S. and Fuzy, E.M. 2006. Virulence of the entomopathogenic nematodes Heterorhabditis bacteriophora, Heterorhabditis zealandica, and Steinernema scarabaei against five white grub species (Coleoptera: Scarabaeidae) of economic importance in turfgrass in North America. Biological Control 38: 397-404.
210. Koppenhofer, A.M., Grewal, P.S. and Fuzy, E.M. 2007. Differences in penetration routes and establishment rates of four entomopathogenic nematode species into four white grub species. Journal of Invertebrate Pathology 94: 184-195.
211. Lee, D.W., Choo, H.Y., Kaya, H.K., Lee, S.M., Smitley, D.R., Shin, H.K. and Park, C.G. 2002. Laboratory and field evaluation of Korean entomopathogenic nematode isolates against the oriental beetle
Exomala orientalis (Coleoptera: Scarabaeidae). Journal of Economic Entomology. 95: 918-926.
212. Li, X.Y., Cowles, R.S., Cowles, E.A., Gaugler, R. and Cox-Foster, D.L. 2007. Relationship between the successful infection by entomopathogenic nematodes and the host immune response. International Journal for Parasitology. 37: 365-374.
213. Mannion, C.M., McLane, W., Klein, M.G., Moyseenko, J., Oliver, J.B. and Cowan D. 2001. Management of early-instar Japanese beetle (Coleoptera: Scarabaeidae) in field-grown nursery crops. Journal of Economic Entomology. 94: 1151-1161.
214. Polavarapu, S., Koppenhoefer, A.M., Barry, J.D., Holdcraft, R.J. and Fuzy, E.M. 2007. Entomopathogenic nematodes and neonicotinoids for remedial control of oriental beetle,
Anomala orientalis (Coleoptera: Scarabaeidae), in highbush blueberry. Crop Protection 26: 1266-1271.
215. Yeh, T. and Alm, S.R. 1995. Evaluation of
Steinernema glaseri (Nematoda: Steinernematidae) for biological control of Japanese and apanese and oriental beetles (Coleoptera, Searabaeidae). Journal of Economic Entomology 88: 1251-1255.
216. Yi, Y.K., Park, H.W., Shrestha, S., Seo, J., Kim, Y.O., Shin, C.S. and Kim, Y. 2007. Identification of two entomopathogenic bacteria from a nematode pathogenic to the oriental beetle,
Blitopertha orientalis. Journal of Microbiology and Biotechnology 17: 968-978.
White grubs, Costelytra zealandica
217. Kain, W.M. Bedding, R.A. and Vandermespel, C.J. 1982. Preliminary evaluations of parasitic nematodes for grass grub (
Costelytra-zealandica (white)) control in central hawkes bay of new-Zealand. New Zealand Journal of Experimental Agriculture 10: 447-450.
White grubs, Cotinus nitida
218.
Koppenhofer, A.M., Fuzy, E.M., Crocker, R.L., Gelernter, W.D. and Polavarapu, S. 2004. Pathogenicity of Heterorhabditis bacteriophora, Steinernema glaseri, and S. scarabaei (Rhabditida: Heterorhabditidae, Steinernematidae) against 12 white grub species (Coleoptera: Scarabaeidae). Biocontrol Science and Technology. 14: 87-92.
219. Townsend, M.L., Johnson, D.T. and Steinkraus, D.C. 1998. Laboratory studies of the interactions of environmental conditions on the susceptibility of green June beetle (Coleoptera: Scarabaeidae) grubs to entomopathogenic nematodes. Journal of Entomological Science 33: 40-48.
220. Townsend, M.L., Steinkraus, D.C. and Johnson, D.T. 1994. Mortality response of green June beetle (Coleoptera, Scarabaeidae) to 4 species of entomopathogenic nematodes. Journal of Entomological Science 29: 268-275.
White grubs, Cyclocephala borealis, C. pasadenae and C. hirta
221.
An, R. and Grewal, P.S. 2007. Differences in the virulence of Heterorhabditis bacteriophora and Steinernema scarabaei to three white grub species: The relative contribution of the nematodes and their symbiotic bacteria. Biological Control 43: 310-316.
222. Converse, V. and Grewal, P.S, 1998. Virulence of entomopathogenic nematodes to the western masked chafer
Cyclocephala hirta (Coleoptera: Scarabaeidae). Journal of Economic Entomology 91: 428-432.
223. Koppenhofer, A.M. and Fuzy, E.M. 2008. Attraction of four entomopathogenic nematodes to four white grub species. Journal of Invertebrate Pathology 99: 227-234.
224. Koppenhofer, A.M. and Fuzy, E.M. 2008. Earl timing and new combinations to increase the efficacy of neonicotinoid-entomopathogenic nematode (Rhabditida: Heterorhabditidae) combinations against white grubs (Coleoptera: Scarabaeidae). Pest Management Science 64: 725-735.
225. Koppenhofer, A.M. and Fuzy, E.M. 2008. Effect of the anthranilic diamide insecticide, chlorantraniliprole, on
Heterorhabditis bacteriophora (Rhabditida: Heterorhabditidae) efficacy against white grubs (Coleoptera: Scarabaeldae). Biological Control 45: 93-102.
226. Koppenhofer, A.M., Choo, H.Y., Kaya, H.K., Lee, D.W. and Gelernter, W.D. 1999. Increased field and greenhouse efficacy against scarab grubs with a combination of an entomopathogenic nematode and
Bacillus thuringiensis. Biological Control 14: 37-44
227. Koppenhofer, A.M., Grewal, P.S. and Fuzy, E.M. 2007. Differences in penetration routes and establishment rates of four entomopathogenic nematode species into four white grub species. Journal of Invertebrate Pathology 94: 184-195.
White grubs, Hoplia philanthus
228.
Ansari, M.A., Adhikari, B.N. and Moens, M. 2008. Susceptibility of Hoplia philanthus (Coleoptera: Scarabaeidae) larvae and pupae to entomopathogenic nematodes (Rhabditida: Steinernematidae, Heterorhabditidae). Biological Control 47: 315-321.
229. Ansari, M.A., Ali, F. and Moens, M. 2006. Compared virulence of the Belgian isolate of
Steinernema glaseri (Rhabditida: Steinernematidae) and the type population of
S-scarabaei to white grub species (Coleoptera: Scarabaeidae). Nematology 8: 787-791.
230. Ansari, M.A., Hussain, M. and Moens, M. 2009. Formulation and application of entomopathogenic nematode-infected cadavers for control of
Hoplia philanthus in turf grass. Pest Management Science. 65: 367-374.
231. Ansari, M.A., Shah, F.A., Tirry, L. and Moens, M. 2006. Field trials against
Hoplia philanthus (Coleoptera: Scarabaeidae) with a combination of an entomopathogenic nematode and the fungus Metarhizium anisopliae CLO 53. Biological Control 39: 453-459.
232. Ansari, M.A., Waeyenberge, L. and Moens, M. 2005. First record of
Steinernema glaseri Steiner, 1929 (Rhabditida: Steinernematidae) from Belgium: a natural pathogen of
Hoplia philanthus (Coleoptera: Scarabaeidae). Nematology 7: 953-956.
White grubs, Melolontha melolontha
233.
Ansari, M.A., Ali, F. and Moens, M. 2006. Compared virulence of the Belgian isolate of Steinernema glaseri (Rhabditida: Steinernematidae) and the type population of S-scarabaei to white grub species (Coleoptera: Scarabaeidae). Nematology 8: 787-791.
234. Malinowski, H. 2011. Possibility of forest protection against insects damaging root systems with the use of biological method based on entomopathogenic nematodes and bacteria. Sylwan 155: 104-111.
235. Steiner, W.A. 1996. Dispersal and host-finding ability of entomopathogenic nematodes at low temperatures. Nematologica 42: 243-261.
White grubs, Ataenius spretulus
236. Alm, S.R., Yeh, T., Hanula, J.L. and Georgis, R. 1992. Biological control of Japanese, oriental and black turfgrass ataenius beetel (Coleoptera, Scarabidae) larvae with entomopathogenic nematodes (Nematoda, Steinernematidae, Heterorhabditidae). Journal of Economic Entomology. 85: 1660-1665.
237.
Koppenhofer, A.M., Fuzy, E.M., Crocker, R.L., Gelernter, W.D. and Polavarapu, S. 2004. Pathogenicity of Heterorhabditis bacteriophora, Steinernema glaseri, and S. scarabaei (Rhabditida: Heterorhabditidae, Steinernematidae) against 12 white grub species (Coleoptera: Scarabaeidae). Biocontrol Science and Technology. 14: 87-92.
White grubs (Asiatic garden beetle), Maladera castanea
238. Koppenhofer, A.M. and Fuzy E.M. 2003. Biological and chemical control of the Asiatic garden beetle,
Maladera castanea (Coleoptera: Scarabaeidae). Journal of Economic Entomology 96: 1076-1082.
239. Koppenhofer, A.M. and Fuzy E.M. 2004. Effect of white grub developmental stage on susceptibility to entomopathogenic nematodes. Journal of Economic Entomology. 97: 1842-1849.
240. Koppenhofer, A.M., Cowles, R.S., Cowles, E.A., Fuzy, E.M. and Baumgartner, L. 2002. Comparison of neonicotinoid insecticides as synergists for entomopathogenic nematodes. Biological Control 24: 90-97.
241. Koppenhofer, A.M., Fuzy, E.M., Crocker, R.L., Gelernter, W.D. and Polavarapu, S. 2004. Pathogenicity of
Heterorhabditis bacteriophora,
Steinernema glaseri, and
S. scarabaei (Rhabditida: Heterorhabditidae, Steinernematidae) against 12 white grub species (Coleoptera: Scarabaeidae). Biocontrol Science and Technology. 14: 87-92.
242.
Koppenhofer, A.M., Grewal, P.S. and Fuzy, E.M. 2006. Virulence of the entomopathogenic nematodes Heterorhabditis bacteriophora, Heterorhabditis zealandica, and Steinernema scarabaei against five white grub species (Coleoptera: Scarabaeidae) of economic importance in turfgrass in North America. Biological Control 38: 397-404.
White grubs, Phyllophaga Spp.
243. Forschler, B.T. and Gardner, W.A. 1991. Concentration mortality response of
Phyllophaga-hirticula (Coleoptera, Scarabaeidae) to 3 entomogenous nematodes. Journal of Economic Entomology 84: 841-843.
244. Forschler, B.T. and Gardner, W.A. 1991. Parasitism of
Phyllophaga-hirticula (coleoptera, scarabaeidae) by
Heterorhabditis-heliothidis and
Steinernema-carpocapsae. Journal of Invertebrate Pathology 58: 396-407.
245. Koppenhofer, A.M., Fuzy, E.M., Crocker, R.L., Gelernter, W.D. and Polavarapu, S. 2004. Pathogenicity of
Heterorhabditis bacteriophora,
Steinernema glaseri, and
S. scarabaei (Rhabditida: Heterorhabditidae, Steinernematidae) against 12 white grub species (Coleoptera: Scarabaeidae). Biocontrol Science and Technology. 14: 87-92.
246.
Koppenhofer, A.M., Rodriguez-Saona, C.R., Polavarapu, S. and Holdcraft, R.J. 2008. Entomopathogenic nematodes for control of Phyllophaga georgiana (Coleoptera: Scarabaeidae) in cranberries. Biocontrol Science and Technology 18: 21-31.
247. Liesch, P.J. and Williamson, R.C. 2010. Evaluation of chemical controls and entomopathogenic nematodes for control of
Phyllophaga white grubs in a Fraser Fir production field. Journal of Economic Entomology 103: 1979-1987.
248. Melo, E.L., Ortega, C.A., Gaigl, A. and Bellotti, A. 2010. Evaluation of entomopathogenic nematodes for the management of
Phyllophaga bicolor (Coleoptera: Melolonthidae). Revista Colombiana de Entomologia 36: 207-212.
249. Melo-Molina, E.L., Ortega-Ojeda, C.A. and Gaigl, A. 2007. The effect of nematodes on larvae of
Phyllophaga menetriesi and
Anomala inconstans (Coleoptera: Melolonthidae). Revista Colombiana de Entomologia 33: 21-26.
250. Nguyen, K.B., and Buss, E.A. 2011.
Steinernema phyllophagae n. sp (Rhabditida: Steinernematidae), a new entomopathogenic nematode from Florida, USA. Nematology 13: 425-442.
White grubs, Rhizotrogus majalis
251. An, R. and Grewal, P.S. 2007. Differences in the virulence of
Heterorhabditis bacteriophora and
Steinernema scarabaei to three white grub species: The relative contribution of the nematodes and their symbiotic bacteria. Biological Control 43: 310-316.
252. An, R.S., Sreevatsan, S. and Grewal, P.S. 2009. Comparative in vivo gene expression of the closely related bacteria
Photorhabdus temperata and
Xenorhabdus koppenhoeferi upon infection of the same insect host,
Rhizotrogus majalis. BMC Genomics. 10: 433.
253. Koppenhofer, A.M. and Fuzy, E.M. 2008. Attraction of four entomopathogenic nematodes to four white grub species. Journal of Invertebrate Pathology 99: 227-234.
254.
Koppenhofer, A.M., Grewal, P.S. and Fuzy, E.M. 2006. Virulence of the entomopathogenic nematodes Heterorhabditis bacteriophora, Heterorhabditis zealandica, and Steinernema scarabaei against five white grub species (Coleoptera: Scarabaeidae) of economic importance in turfgrass in North America. Biological Control 38: 397-404.
255. Koppenhofer, A.M., Grewal, P.S. and Fuzy, E.M. 2007. Differences in penetration routes and establishment rates of four entomopathogenic nematode species into four white grub species. Journal of Invertebrate Pathology 94: 184-195.
Fuller rose beetle, Asynonychus godmani
256. Morse, J.G. and Lindegren, J.E. 1996. Suppression of fuller rose beetle (Coleoptera: Curculionidae) on citrus with
Steinernema carpocapsae (Rhabditida: Steinernematidae). Florida Entomologist 79: 373-384.
Chive gnat, Bradysia odoriphaga
257. Ma, J., Chen, S.L., Moens, M., Han, R.C. and De Clercq, P. 2013. Efficacy of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) against the chive gnat,
Bradysia odoriphaga. Journal of Pest Science 86: 551-561.
258. Sun, R. H., A. H. Li, R. C. Han, L. Cao, and X. L. Liu. 2004. Factors affecting the control of
Bradysia odoriphaga with entomopathogenic nematode
Heterorhabditis indica LN2. Natural Enemies of Insects 26:150–155.
 [/caption]
[/caption]
 [/caption]
Therefore, these nematodes are also recognized and sold as beneficial nematodes. Unlike toxic chemical nematicides/pesticides, these beneficial nematodes are safe to the environment, human health, both pet and wild animals, and plants. Also, they are not harmful to beneficial insects such as honeybees. Therefore, in this blog, we are providing some basic information on the mutualistic association between nematodes and their symbiotic bacteria, life cycle, host finding ability, production and application of entomopathogenic nematodes. Also, in our routine blog articles, we would like to provide a description of different insect and mollusk pests and their susceptibility to different species of entomopathogenic nematodes.
[/caption]
Therefore, these nematodes are also recognized and sold as beneficial nematodes. Unlike toxic chemical nematicides/pesticides, these beneficial nematodes are safe to the environment, human health, both pet and wild animals, and plants. Also, they are not harmful to beneficial insects such as honeybees. Therefore, in this blog, we are providing some basic information on the mutualistic association between nematodes and their symbiotic bacteria, life cycle, host finding ability, production and application of entomopathogenic nematodes. Also, in our routine blog articles, we would like to provide a description of different insect and mollusk pests and their susceptibility to different species of entomopathogenic nematodes.
 [/caption]
[/caption]
 [/caption]
[caption id="attachment_691" align="aligncenter" width="300" caption="Fig. 5. Red colored Heterorhabditis nematode infected wax worm cadavers"]
[/caption]
[caption id="attachment_691" align="aligncenter" width="300" caption="Fig. 5. Red colored Heterorhabditis nematode infected wax worm cadavers"] [/caption]
Both heterorhabditid and steinernematid nematodes follow two slightly different reproduction pathways. For example, the first generation individuals of heterorhabditid nematodes are produced by self-fertile hermaphrodites (hermaphroditic) and their succeeding generations are produced by cross fertilization between males and females called as amphimictic type of reproduction. In case of Steinernematid nematodes, with an exception of Steinernema hermaphroditum (Griffin et al., 2001; Stock et al., 2004), all generations are produced by cross fertilization between males and females. At the beginning eggs laid by females or hermaphrodites hatch and juveniles start feeding on the cadaver body tissue and bacterial soup. However, old females or hermaphrodites later do not lay eggs, which generally hatch only in the uterus of females. The hatched juveniles then start feeding on the mother’s tissues, the process is termed as “endotokia matricida” (Fig. 6; Johnigk and Ehlers, 1999).
[caption id="attachment_447" align="aligncenter" width="300" caption="Fig. 6. After hatching from the eggs in the uterus, juveniles start feeding on mother’s tissues and this process is termed as Endotokia matricida"]
[/caption]
Both heterorhabditid and steinernematid nematodes follow two slightly different reproduction pathways. For example, the first generation individuals of heterorhabditid nematodes are produced by self-fertile hermaphrodites (hermaphroditic) and their succeeding generations are produced by cross fertilization between males and females called as amphimictic type of reproduction. In case of Steinernematid nematodes, with an exception of Steinernema hermaphroditum (Griffin et al., 2001; Stock et al., 2004), all generations are produced by cross fertilization between males and females. At the beginning eggs laid by females or hermaphrodites hatch and juveniles start feeding on the cadaver body tissue and bacterial soup. However, old females or hermaphrodites later do not lay eggs, which generally hatch only in the uterus of females. The hatched juveniles then start feeding on the mother’s tissues, the process is termed as “endotokia matricida” (Fig. 6; Johnigk and Ehlers, 1999).
[caption id="attachment_447" align="aligncenter" width="300" caption="Fig. 6. After hatching from the eggs in the uterus, juveniles start feeding on mother’s tissues and this process is termed as Endotokia matricida"] [/caption]
Depending on availability of food resources, both the heterorhabditid and steinernematid nematodes generally complete 2-3 generations within insect cadaver and emerge as infective juveniles to seek new hosts. Generally, life cycle of entomopathogenic nematodes starting from the penetration of infective juvenile into their hosts to the emergence of the infective juvenile from host cadavers is completed within 12- 15 days at room temperature (Fig. 7; adopted from http://www.nematodeinformation.com). The optimum temperature for growth and reproduction of most of the entomopathogenic nematode species is between 25 and 30oC (Grewal et al., 1994).
[caption id="attachment_674" align="aligncenter" width="300" caption="Fig. 7. Life cycle of entomopathogenic nematodes. Adopted from www.nematodeinformation.com Click on a image for its enlargement."]
[/caption]
Depending on availability of food resources, both the heterorhabditid and steinernematid nematodes generally complete 2-3 generations within insect cadaver and emerge as infective juveniles to seek new hosts. Generally, life cycle of entomopathogenic nematodes starting from the penetration of infective juvenile into their hosts to the emergence of the infective juvenile from host cadavers is completed within 12- 15 days at room temperature (Fig. 7; adopted from http://www.nematodeinformation.com). The optimum temperature for growth and reproduction of most of the entomopathogenic nematode species is between 25 and 30oC (Grewal et al., 1994).
[caption id="attachment_674" align="aligncenter" width="300" caption="Fig. 7. Life cycle of entomopathogenic nematodes. Adopted from www.nematodeinformation.com Click on a image for its enlargement."] [/caption]
[/caption]
 [/caption]
[/caption]
 [/caption]
[/caption]
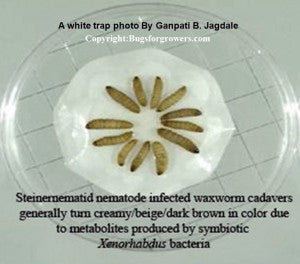 [/caption]
[caption id="attachment_477" align="aligncenter" width="300" caption="Fig. 11. A White trap containing entomopathogenic Heterorhabditis nematode infected wax worm larval cadavers. Click the image for its enlargement"]
[/caption]
[caption id="attachment_477" align="aligncenter" width="300" caption="Fig. 11. A White trap containing entomopathogenic Heterorhabditis nematode infected wax worm larval cadavers. Click the image for its enlargement"] [/caption]
[/caption]


