1. What are insect-parasitic/entomopathogenic nematodes?
By definition nematodes are thread-like microscopic, colorless and unsegmented
round worms found in almost all habitats especially soil and water (Fig. 1).
[caption id="attachment_338" align="aligncenter" width="176" caption="Fig. 1. Nematodes are microscopic, non-segmented, thread-like round worms. Click on image for enlargement"]

[/caption]
Insect-parasitic nematodes:
Nematodes that infect and complete their development, and reproduction at their insect host's expense are called as insect-parasitic nematodes. In the phylum Nematoda, some members of a family Mermithidae (Order: Mermithida) including mosquito-parasitic nematode,
Romanomermis culicivorax and grasshopper nematode
Mermis nigrescens are considered as insect-parasitic nematodes but not as entomomopathogenic nematodes whereas the members of the two families Steinernematidae and Heterorhabditidae (Order: Rhabditida) including
Steinernema spp. and
Heterorhabditis spp., respectively are considered as both insect-parasitic and entomomopathogenic nematodes.
Entomopathogenic nematodes:
Members of both Steinernematidae and Heterorhabditidae families are also called as entomopathogenic nematodes because their infective juveniles are mutualistically associated with a specific kind of symbiotic bacteria, which are pathogenic to a variety of their insect hosts (
Table 2).
Although entomopathogenic nematodes are naturally present in the soil and responsible for suppressing the natural populations of insect pests, currently the main interest in them is to apply them inundatively as
beneficial biological control agents to manage various economically important insect pests of different agricultural and horticultural crops, and ornamental plants (Grewal et al., 2005). Within last 30-40 years, 26 and 75 different species of Heterorhabditid (
Table 3) and Steinernematid (
Table 4) nematodes, respectively have been isolated and described from various parts of the world. A few of these described nematode species have been commercially produced and used as effective biological control agents against many insect pests of several economically important crops. These nematodes can infect and kill larvae/ caterpillars, pupae and adults of a variety of insect pests (
Table 2; Fig. 2).
[caption id="attachment_704" align="aligncenter" width="300" caption="Fig. 2. Diagram showing that the entomopathogenic nematodes can infect and kill various stages (larvae, pupae and adults) of their host insects."]

[/caption]
Therefore, these nematodes are also recognized and sold as
beneficial nematodes. Unlike toxic chemical nematicides/pesticides, these beneficial nematodes are safe to the environment, human health, both pet and wild animals, and plants. Also, they are not harmful to beneficial insects such as
honeybees. Therefore, in this blog, we are providing some basic information on the mutualistic association between nematodes and their symbiotic bacteria, life cycle, host finding ability, production and application of entomopathogenic nematodes. Also, in our routine blog articles, we would like to provide a description of different insect and mollusk pests and their susceptibility to different species of entomopathogenic nematodes.
2. What kinds of symbiotic bacteria are associated with entomopathogenic nematodes?
- Two different kinds of symbiotic bacteria in the genus, Photorhabdus (Table 3) and Xenorhabdus (Table 4) are symbiotically associated with the species specific infective juveniles of Heterorhabditis spp. (Family: Heterorhabditidae) and Steinernema spp. (Family: Steinernematidae), respectively.
- Species of both Xenorhabdus and Photorhabdus are motile gram-negative bacteria belong to the family Enterobacteriaceae and also exist in two main phenotypic forms (phase I and II), a phenomenon known as phase variation (Han and Ehlers, 2001).
- The phase I form (also termed as primary form) varies physiologically and morphologically from phase II form (also called as secondary form).
- Also, a main property distinguishing Xenorhabdus spp. from Photorhabdus spp. is that the only Photorhabdus bacteria have an ability to emit the light under stationary-phase culture conditions and in the infected host insect cadavers.
3. What is an infective juvenile?
A third-stage juvenile of an entomopathogenic nematode is called as an infective juvenile because it initiates the infection in its host. Infective juvenile is the only non-feeding and free-living stage found in the soil but all other stages including fourth and fifth (adult) and egg stages are completed inside the host.
4. What is a dauer juvenile?
The infective juveniles are actually third-stage juvenile that also called as dauer juveniles because they are enclosed in a second-stage cuticle, which arrests their further development (Fig.3; adopted from
http://www.nematodeinformation.com) and helps to survive outside the host i.e. in the soil environment. Furthermore, these developmentally arrested dauer juveniles are physiologically adapted to remain in the environment (i.e. soil) without feeding until a perspective host is located. These dauer juveniles recover and resume their development only when they enter the perspective insect host’s body cavity via natural openings and shed their second stage cuticle. The dauer juveniles are also well known to tolerate harsh environmental conditions including extreme hot and cold temperatures, and desiccation (Jagdale and Gordon, 1997; Jagdale and Grewal, 2003; 2007; Jagdale et a., 2005).
[caption id="attachment_470" align="aligncenter" width="300" caption="Fig. 3. A dauer juvenile of an entomopathogenic Steinernema carpocapsae nematode. adapted from www.nematodeinformation.com. Click the image for its enlargement"]

[/caption]
5. Life cycle of entomopathogenic nematodes
As stated above, entomopathogenic nematodes complete most of their life cycle inside insect cadavers with an exception of infective/dauer juvenile, the only free-living stage found in the environment i.e. in the soil. Both
Steinernema and
Heterorhabditis infective juveniles locate an insect host and enter its body
through natural body openings such as mouth, anus or spiracles. In addition, infective juveniles of Heterorhabditis species can also enter through the inter-segmental members of the host cuticle.
Infective juveniles then actively penetrate through the mid-gut wall or tracheae into the insect body cavity also called hemocoel, which is filled with the insect blood also termed as haemolymph. Once in the hemocoel, infective juveniles release symbiotic bacteria from their intestine through anus in the insect haemolymph. Bacteria start multiplying in the nutrient-rich haemolymph and infective juveniles recover from their arrested state (dauer stage) and start feeding on multiplying bacteria and disintegrated host tissues. Toxins produced by the developing nematodes and multiplying bacteria in the body cavity kill the insect host usually within 48 hours.These bacteria also produce a plethora of metabolites, toxins and antibiotics with bactericidal, fungicidal and nematicidal properties, which ensures monoxenic conditions for nematode development and reproduction in the insect cadaver. Generally, if insect hosts such as wax worm larvae are infected with Steinernematid nematodes, they will turn creamy/beige/dark brown in color due to the metabolites produced by their symbiotic
Xenorhabdus bacteria (Figs. 4 & 10) and if they are infected with Heterorhabditid nematodes, they will turn reddish/purplish in color to the metabolites produced by their symbiotic
Photorhabdus bacteria (Figs. 5 & 11).
[caption id="attachment_690" align="aligncenter" width="300" caption="Fig. 4. Beig colored Steinernematid nematode infected wax worm cadavers"]

[/caption]
[caption id="attachment_691" align="aligncenter" width="300" caption="Fig. 5. Red colored Heterorhabditis nematode infected wax worm cadavers"]

[/caption]
Both heterorhabditid and steinernematid nematodes follow two slightly different reproduction pathways. For example, the first generation individuals of heterorhabditid nematodes are produced by self-fertile hermaphrodites (hermaphroditic) and their succeeding generations are produced by cross fertilization between males and females called as amphimictic type of reproduction. In case of Steinernematid nematodes, with an exception of
Steinernema hermaphroditum (Griffin et al., 2001; Stock et al., 2004), all generations are produced by cross fertilization between males and females. At the beginning eggs laid by females or hermaphrodites hatch and juveniles start feeding on the cadaver body tissue and bacterial soup. However, old females or hermaphrodites later do not lay eggs, which generally hatch only in the uterus of females. The hatched juveniles then start feeding on the mother’s tissues, the process is termed as “endotokia matricida” (Fig. 6; Johnigk and Ehlers, 1999).
[caption id="attachment_447" align="aligncenter" width="300" caption="Fig. 6. After hatching from the eggs in the uterus, juveniles start feeding on mother’s tissues and this process is termed as Endotokia matricida"]

[/caption]
Depending on availability of food resources, both the heterorhabditid and steinernematid nematodes generally complete 2-3 generations within insect cadaver and emerge as infective juveniles to seek new hosts. Generally, life cycle of entomopathogenic nematodes starting from the penetration of infective juvenile into their hosts to the emergence of the infective juvenile from host cadavers is completed within 12- 15 days at room temperature (Fig. 7; adopted from
http://www.nematodeinformation.com). The optimum temperature for growth and reproduction of most of the entomopathogenic nematode species is between 25 and 30
oC (Grewal et al., 1994).
[caption id="attachment_674" align="aligncenter" width="300" caption="Fig. 7. Life cycle of entomopathogenic nematodes. Adopted from www.nematodeinformation.com Click on a image for its enlargement."]

[/caption]
6. How do entomopathogenic nematodes locate their insect hosts?
Entomopathogenic nematode infective juveniles use following three types of foraging strategies to locate their insect hosts.
a. Ambush foraging:
Some entomopathogenic nematodes like
Steinernema carpocapsae and
S. scapterisci have adapted ambush foraging behavior known as “sit and wait” strategy to attack highly mobile insects including billbugs, sod webworms, cutworms, mole-crickets and armyworms at the surface of the soil. These nematodes do not respond to host released cues but infective juveniles of some
Steinernema spp can stand on their tails (nictate) and easily infect passing insect hosts by jumping on them. Since highly mobile insects live in the upper soil or thatch layer, ambushers are generally effective in infecting more insects on the surface than deep in the soil.
b. Cruise foraging:
Cruiser entomomatogenic nematodes such as
Heterorhabditis bacteriophora, H. megidis, Steinernema glaseri and
S. kraussei are generally move actively in search of hosts and therefore, they found throughout the soil profile and more effective against less mobile hosts such as white grubs and larvae of black vine weevils. These cruisers never nictate but generally respond to carbon dioxide released by insect hosts as cues.
c. Intermediate foraging:
Some entomopathogenic nematode species such as
Steinernema feltiae and
S.riobrave have adapted a foraging behavior that lie in between ambush and cruise strategies called an intermediate strategy to attack both the mobile and sedentary/less mobile insects at the surface or immobile stages deep in the soil.
Steinernema feltiae is highly effective against fungus gnats and mushroom flies whereas
S.riobrave is effective against corn earworms, citrus root weevils and mole crickets.
7. How are entomopathogenic nematodes produced?
Currently, two different techniques including
in vivo and
in vitro are used for the mass production of entomopathogenic nematodes (Ehlers and Shapiro-Ilan, 2005). Generally for a small-scale nematode production,
in vivo technique is used whereas for a large-scale nematode production
in vitro technique is used. In
in vivo production technique, the nematode production is carried out in insect hosts; most commonly in last instar larvae of wax worms,
Galleria mellonella (Fig. 8 ) or mealworms,
Tenebrio molitor whereas
in vitro production is carried out in solid or liquid media. Since in vitro technique is costly, needs a large infrastructure and installation, a thorough knowledge of bioreactor technology and biology of both entomopathogenic nematodes and their symbiotic bacteria, this blog focuses only on
in vivo nematode production technique. For more information on
in vitro nematode production technology read a book chapter by Ehlers and Shapiro-Ilan (2005).
[caption id="attachment_455" align="aligncenter" width="300" caption="Fig. 8. Fourth stage wax worm Galleria melonella larvae used for in vio production of entomopathogenic nematodes."]

[/caption]
In vivo production of entomopathogenic nematodes:
Briefly, in this technique insect host larvae are inoculated with infective juveniles of entomopathogenic nematodes in dishes or in trays lined with a filter paper or any other available absorbent substrate (Fig. 9). For effective infection and optimum production, about 100 infective juveniles are used for infection of each wax worm or mealworm larva. The filter papers are generally used in dishes for absorption of excess nematode suspension so that insect larvae are not drowned in the suspension and infective juveniles can easily find moving insect host larvae for infection. Insects will die within 48 hours of infection (Figs. 4 and 5). After 48- 72 hours, the insect larval cadavers are transferred to the White traps (see below Figs. 10 and 11; White 1927). These white traps are then held in an incubator for 10-12 days at optimum temperature ranging from 18 to 28
oC (Grewal et al., 1994). After 10-12 days into white traps, infective juveniles of entomopathogenic nematode generally start emerging from cadavers and moving into water. Emerged infective juveniles are then harvested from White traps, cleaned and concentrated by gravity settling (Dutky et al., 1964). These cleaned nematodes are ready for field applications or laboratory use.
[caption id="attachment_696" align="aligncenter" width="300" caption="Fig. 9. A Petri dish lined with a filter paper for infecting insects with entomopathogenic nematodes."]

[/caption]
8. How to make a White trap?
For making White traps, you need one large size dish, a bottom or lid of a small size dish and a filter paper. As shown in Figs. 10 and 11, place a bottom or lid of a small dish inside the large size dish. Cover the bottom or lid of a small dish with a filter paper and then arrange cadavers on the filter paper. Then add enough quantity of water into large dish making sure that the filter paper is touching to water and becoming wet. Replace the lid of large dish and transfer into an incubator for 10-12 days. After 10-12 days, infective juveniles of entomopathogenic nematodes will emerge from cadavers and move into water.
[caption id="attachment_476" align="aligncenter" width="300" caption="Fig. 10. A White trap containing entomopathogenic Steinernematid nematode infected wax worm larval cadavers. Click the image for its enlargement"]
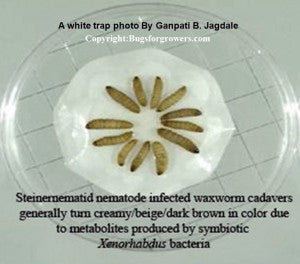
[/caption]
[caption id="attachment_477" align="aligncenter" width="300" caption="Fig. 11. A White trap containing entomopathogenic Heterorhabditis nematode infected wax worm larval cadavers. Click the image for its enlargement"]

[/caption]
9. What are similarities and differences between Steinernematid and Heterorhabditid nematodes?
Similarities:
Characteristics
|
Steinernematid Nematodes
|
Heterorhabditid Nematodes
|
| A single free-living and non-feeding infective/ dauer juvenile stage
|
Present |
Present |
| Infective juveniles carry several cells of symbiotic bacterial in their guts |
Yes |
Yes |
| Infective juveniles enter into insect host’s body cavity through natural openings such as mouth, spiracles and anus |
Yes |
Yes |
| Once in the body cavity, symbiotic bacteria released by infective juveniles into insect blood through anus |
Yes |
Yes |
| In insect blood, symbiotic bacteria quickly multiply, cause a disease and kill insect host within 48 hours of nematode infection (Griffin et al., 2005) |
Yes |
Yes |
Differences:
Characteristics
|
Steinernematid Nematodes
|
Heterorhabditid Nematodes
|
| Taxonomic relationship (Stock and Hunt, 2005) |
No close relationship |
No close relationship |
| Type of reproduction (Griffin et al., 2005) |
Amphimictic reproduction: All generations are produced by a cross fertilization between males and females |
Both hermaphrodictic and amphimictic reproductions: In hermphrodictic reproduction, first generation individuals are produce by self-fertilization i.e. without males but the second generation individuals are produced by following amphimictic type of reproduction.
|
| Number of infective juveniles need to enter into insect host’s body |
At least two infective juveniles need to develop into a separate male and female individual for cross-fertilization and colonization |
Only one infective juveniles need to develop as a hermaphrodite. |
| Type of symbiotic bacteria carried by infective juveniles |
Xenorhabdus spp. |
Photorhabdus spp. |
10. Why are entomopathogenic nematodes excellent and safe biological control agents?
Entomopathogenic nematodes also called as beneficial nematodes belonging to both families, Steinernematidae and Heterorhabditidae are considered as safe and excellent biological control agents against many soil dwelling insect pests (Table 2) of many economically important crops because…..
- they have a broad host range
- their ability to search actively for hosts
- their ability to kill their hosts rapidly within 24-48 hours
- they have potential to recycle in the soil environment
- they have no deleterious effects on humans, other vertebrate animals, non-target organisms and plants
- they have no negative effects on environment
- they can be easily mass produced using both in vivo and in vitro methods and applied using traditional insecticide spraying equipments
- they are compatible with many chemical insecticides and biopesticides and therefore, easily included in IPM programs
- there is no fear of developing resistance in their insect hosts as these nematodes physically enter into the insect host's body cavity where they release symbiotically associated bacteria and kill insect host within 48 hours.
- Because of their safety to the environment and human health, they also been exempted from registration and regulation requirement by US Environmental Protection Agency (EPA) and similar agencies in many other countries
11. How many nematodes do I need to apply for the successful control of target pests?
For the successful control of most of the soil dwelling insect pests, the optimal rate of
1 billion infective juvenile nematodes in 100 to 260 gallons of water
per acre is generally recommended (See
Table 1).
12. How are entomopathogenic nematodes applied?
Please read
our previous blog for appropriate methods of nematode application.
References
Dutky, S. R., Thompson, J. V. and Cantwell, G. E. 1964. A technique for the mass propagation of the DD-136 nematode. Journal of Insect Pathology 6, 417- 422.
Ehlers, R.-U. and Shapiro-Ilan, D. I. 2005. Mass production. In: Nematodes as biocontrol agents. Grewal, P. S., Ehlers, R.-U. and Shapiro-Ilan, D. I. (Eds.). CABI Publishing,UK. pp. 65-78.
Grewal, P.S., Ehlers, R.U. and Shapiro-Ilan, D. I. [Editors]. 2005. Nematodes As Biocontrol Agents, CABI Publishing, Wallingford, UK, pp 1-505.
Grewal, P.S., Selvan, S., Gaugler, R., 1994. Thermal adaptation of entomopathogenic nematodes: Niche breadth for infection, establishment, and reproduction. J. Therm. Biol. 19, 245-53.
Griffin, C.T., Boemare, N.E. and Lewis, E.E. Biology and behaviour. In: Nematodes as biocontrol agents. Grewal, P. S., Ehlers, R.-U. and Shapiro-Ilan, D. I. (Eds.). CABI Publishing, UK. pp. 47-64.
Griffin, C.T., O'Callaghan, K.M. and Dix, I. 2001. A self-fertile species of
Steinernema from Indonesia: further evidence of convergent evolution amongst entomopathogenic nematodes? Parasitology 122: 181-186.
Han, R. and Ehlers, R. 2001. Effect of
Photorhabdus luminescens phase variants on the in vivo and in vitro development and reproduction of the entomopathogenic nematodes
Heterorhabditis bacteriophora and
Steinernema carpocapsae. FEMS Microbiological Ecology 35: 239-247.
Jagdale, G.B. and Gordon, R. 1997.
Effect of temperature on the composition of fatty acids in total lipids and phospholipids of entomopathogenic nematodes. Journal of Thermal Biology
22
: 245-251.
Jagdale, G.B. and Grewal, P.S. 2003. Acclimation of entomopathogenic nematodes to novel temperatures: trehalose accumulation and the acquisition of thermotolerance. International Journal for Parasitology
33: 145-152.
Jagdale, G. B. and Grewal, P. S. 2007. Storage temperature influences desiccation and ultra violet radiation tolerance of entomopathogenic nematodes. Journal of Thermal Biology 32: 20-27.
Jagdale, G. B
., Grewal, P. S. and Salminen, S. O. 2005. Both heat-shock and cold-shock influence trehalose metabolism in entomopathogenic nematodes. Journal of Parasitology 91
: 988-994.
Johnigk, S.-A., and Ehlers, R.-U. 1999.
Endotokia matricida in hermaphrodites of
Heterorhabditis spp and the effect of the food supply. Nematology 1, 717–726.
Stock, S. P. and Hunt, D. J., 2005, Morphology and systematics of nematodes used in biocontrol. In: Nematodes as biocontrol agents. Grewal, P. S., Ehlers, R.-U. and Shapiro-Ilan, D. I. (Eds.). CABI Publishing,UK. pp. 3-43.
Stock, S.P., Griffin, C.T., and Haerani, R.C. 2004. Morphological and molecular characterization of Steinernema hermaphroditum n. sp. (Nematoda: Steinernematidae), an entomopathogenic nematode from Indonesia, and its phylogenetic relationships with other members of the genus. Nematology 6: 401- 412.
White, G.F., 1927. A method for obtaining infective nematode larvae from cultures. Science 66, 302-303.
 [/caption]
[/caption]
 [/caption]
Therefore, these nematodes are also recognized and sold as beneficial nematodes. Unlike toxic chemical nematicides/pesticides, these beneficial nematodes are safe to the environment, human health, both pet and wild animals, and plants. Also, they are not harmful to beneficial insects such as honeybees. Therefore, in this blog, we are providing some basic information on the mutualistic association between nematodes and their symbiotic bacteria, life cycle, host finding ability, production and application of entomopathogenic nematodes. Also, in our routine blog articles, we would like to provide a description of different insect and mollusk pests and their susceptibility to different species of entomopathogenic nematodes.
[/caption]
Therefore, these nematodes are also recognized and sold as beneficial nematodes. Unlike toxic chemical nematicides/pesticides, these beneficial nematodes are safe to the environment, human health, both pet and wild animals, and plants. Also, they are not harmful to beneficial insects such as honeybees. Therefore, in this blog, we are providing some basic information on the mutualistic association between nematodes and their symbiotic bacteria, life cycle, host finding ability, production and application of entomopathogenic nematodes. Also, in our routine blog articles, we would like to provide a description of different insect and mollusk pests and their susceptibility to different species of entomopathogenic nematodes.
 [/caption]
[/caption]
 [/caption]
[caption id="attachment_691" align="aligncenter" width="300" caption="Fig. 5. Red colored Heterorhabditis nematode infected wax worm cadavers"]
[/caption]
[caption id="attachment_691" align="aligncenter" width="300" caption="Fig. 5. Red colored Heterorhabditis nematode infected wax worm cadavers"] [/caption]
Both heterorhabditid and steinernematid nematodes follow two slightly different reproduction pathways. For example, the first generation individuals of heterorhabditid nematodes are produced by self-fertile hermaphrodites (hermaphroditic) and their succeeding generations are produced by cross fertilization between males and females called as amphimictic type of reproduction. In case of Steinernematid nematodes, with an exception of Steinernema hermaphroditum (Griffin et al., 2001; Stock et al., 2004), all generations are produced by cross fertilization between males and females. At the beginning eggs laid by females or hermaphrodites hatch and juveniles start feeding on the cadaver body tissue and bacterial soup. However, old females or hermaphrodites later do not lay eggs, which generally hatch only in the uterus of females. The hatched juveniles then start feeding on the mother’s tissues, the process is termed as “endotokia matricida” (Fig. 6; Johnigk and Ehlers, 1999).
[caption id="attachment_447" align="aligncenter" width="300" caption="Fig. 6. After hatching from the eggs in the uterus, juveniles start feeding on mother’s tissues and this process is termed as Endotokia matricida"]
[/caption]
Both heterorhabditid and steinernematid nematodes follow two slightly different reproduction pathways. For example, the first generation individuals of heterorhabditid nematodes are produced by self-fertile hermaphrodites (hermaphroditic) and their succeeding generations are produced by cross fertilization between males and females called as amphimictic type of reproduction. In case of Steinernematid nematodes, with an exception of Steinernema hermaphroditum (Griffin et al., 2001; Stock et al., 2004), all generations are produced by cross fertilization between males and females. At the beginning eggs laid by females or hermaphrodites hatch and juveniles start feeding on the cadaver body tissue and bacterial soup. However, old females or hermaphrodites later do not lay eggs, which generally hatch only in the uterus of females. The hatched juveniles then start feeding on the mother’s tissues, the process is termed as “endotokia matricida” (Fig. 6; Johnigk and Ehlers, 1999).
[caption id="attachment_447" align="aligncenter" width="300" caption="Fig. 6. After hatching from the eggs in the uterus, juveniles start feeding on mother’s tissues and this process is termed as Endotokia matricida"] [/caption]
Depending on availability of food resources, both the heterorhabditid and steinernematid nematodes generally complete 2-3 generations within insect cadaver and emerge as infective juveniles to seek new hosts. Generally, life cycle of entomopathogenic nematodes starting from the penetration of infective juvenile into their hosts to the emergence of the infective juvenile from host cadavers is completed within 12- 15 days at room temperature (Fig. 7; adopted from http://www.nematodeinformation.com). The optimum temperature for growth and reproduction of most of the entomopathogenic nematode species is between 25 and 30oC (Grewal et al., 1994).
[caption id="attachment_674" align="aligncenter" width="300" caption="Fig. 7. Life cycle of entomopathogenic nematodes. Adopted from www.nematodeinformation.com Click on a image for its enlargement."]
[/caption]
Depending on availability of food resources, both the heterorhabditid and steinernematid nematodes generally complete 2-3 generations within insect cadaver and emerge as infective juveniles to seek new hosts. Generally, life cycle of entomopathogenic nematodes starting from the penetration of infective juvenile into their hosts to the emergence of the infective juvenile from host cadavers is completed within 12- 15 days at room temperature (Fig. 7; adopted from http://www.nematodeinformation.com). The optimum temperature for growth and reproduction of most of the entomopathogenic nematode species is between 25 and 30oC (Grewal et al., 1994).
[caption id="attachment_674" align="aligncenter" width="300" caption="Fig. 7. Life cycle of entomopathogenic nematodes. Adopted from www.nematodeinformation.com Click on a image for its enlargement."] [/caption]
[/caption]
 [/caption]
[/caption]
 [/caption]
[/caption]
 [/caption]
[caption id="attachment_477" align="aligncenter" width="300" caption="Fig. 11. A White trap containing entomopathogenic Heterorhabditis nematode infected wax worm larval cadavers. Click the image for its enlargement"]
[/caption]
[caption id="attachment_477" align="aligncenter" width="300" caption="Fig. 11. A White trap containing entomopathogenic Heterorhabditis nematode infected wax worm larval cadavers. Click the image for its enlargement"] [/caption]
[/caption]
